Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Molecular orbital theory
Molecular
orbital theory
Lewis
concept and valence bond theory qualitatively explains the chemical bonding and
molecular structure. Both approaches are inadequate to describe some of the
observed properties of molecules. For example, these theories predict that
oxygen is diamagnetic. However, it was observed that oxygen in liquid form was
attracted towards the poles of strong magnet, indicating that oxygen is
paramagnetic. As both these theories treated the bond formation in terms of
electron pairs and hence they fail to explain the bonding nature of
paramagnetic molecules. F. Hund and Robert. S. Mulliken developed a bonding
theory called molecular orbital theory which explains the magnetic behaviour of
molecules.
The salient features of this theory are as follows.
1.
When atoms combines to form molecules, their individual atomic orbitals lose
their identity and forms new orbitals called molecular orbitals.
2.
The shapes of molecular orbitals depend upon the shapes of combining atomic
orbitals.
3.
The number of molecular orbitals formed is the same as the number of combining
atomic orbitals. Half the number of molecular orbitals formed will have lower
energy than the corresponding atomic orbital, while the remaining molecular
orbitals will have higher energy. The molecular orbital with lower energy is
called bonding molecular orbital and the one with higher energy is called
anti-bonding molecular orbital. The bonding molecular orbitals are represented
as σ (Sigma), π (pi), δ (delta) and the corresponding antibonding orbitals are
denoted as σ*, π* and δ*.
4.
The electrons in a molecule are accommodated in the newly formed molecular
orbitals. The filling of electrons in these orbitals follows Aufbau's
principle, Pauli's exclusion principle and Hund's rule as in the case of
filling of electrons in atomic orbitals.
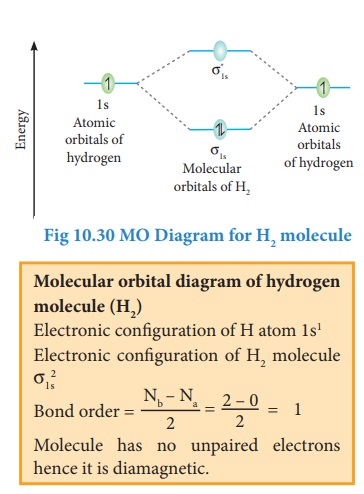
5.
Bond order gives the number of covalent bonds between the two combining atoms.
The bond order of a molecule can be calculated using the following equation
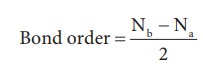
Where,
Nb
= Total number of electrons present in the bonding molecular orbitals
Na
= Total number of electrons present in the antibonding molecular orbitals and
A
bond order of zero value indicates that the molecule doesn't exist.
Linear combination of atomic orbitals
The
wave functions for the molecular orbitals can be obtained by solving
Schrödinger wave equation for the molecule. Since solving the Schrödinger
equation is too complex, approximation methods are used to obtain the wave
function for molecular orbitals. The most common method is the linear
combination of atomic orbitals (LCAO).
We
know that the atomic orbitals are represented by the wave function Ψ. Let us
consider two atomic orbitals represented by the wave function ψA and
ψB with comparable energy, combines to form two molecular orbitals.
One is bonding molecular orbital(ψbonding) and the other is
antibonding molecular orbital(ψantibonding). The wave functions for
these two molecular orbitals can be obtained by the linear combination of the
atomic orbitals ψA and ψB as below.
ψbonding
= ψA + ψB
ψantibonding
= ψA - ψB
The formation of bonding molecular orbital can be considered as the result of constructive interference of the atomic orbitals and the formation of anti-bonding molecular orbital can be the result of the destructive interference of the atomic orbitals. The formation of the two molecular orbitals from two 1s orbitals is shown below.
Constructive
interaction: The two 1s orbitals are in phase and have the same sign.
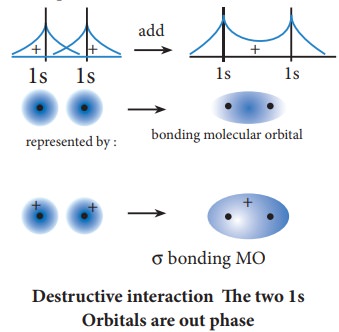
Destructive interaction The two 1s Orbitals
are out phase
Bonding in some Homonuclear di-atomic molecules:
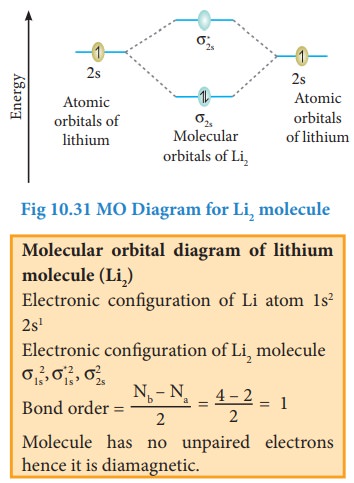
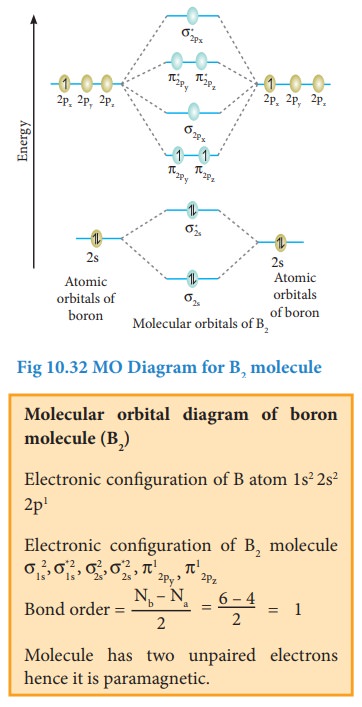
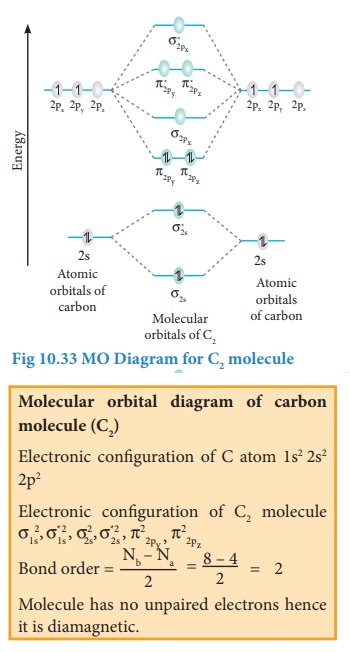
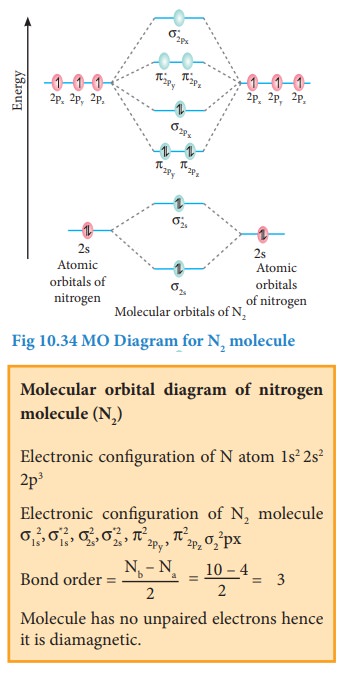
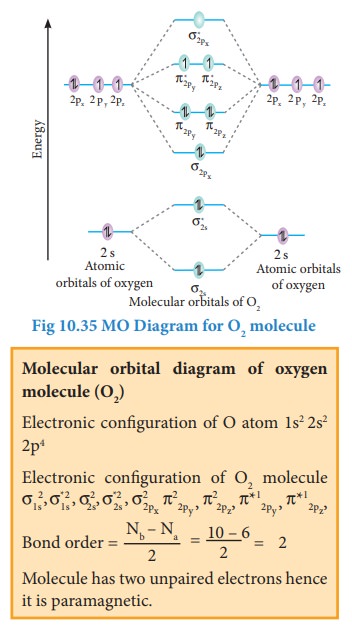
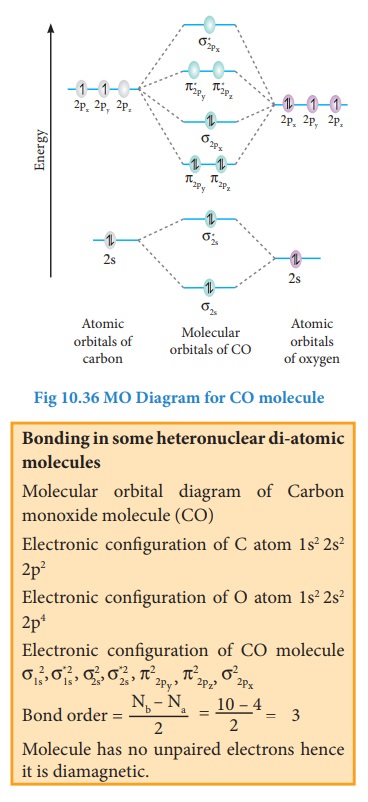
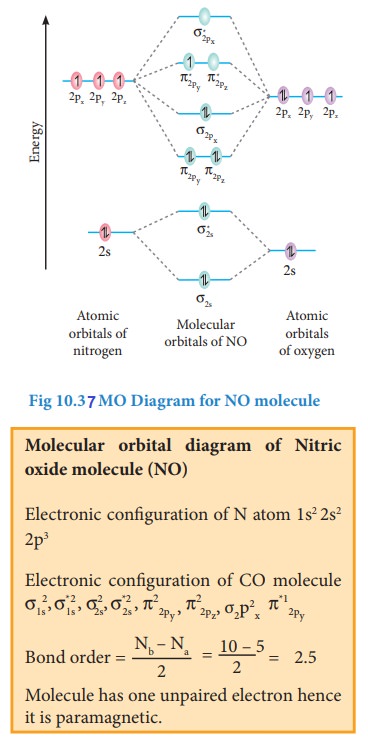
Metallic bonding
Metals
have some special properties of lustre, high density, high electrical and
thermal conductivity, malleability and ductility, and high melting and boiling
points. The forces that keep the atoms of the metal so closely in a metallic
crystal constitute what is generally known as the metallic bond. The metallic
bond is not just an electrovalent bond(ionic bond), as the latter is formed
between atoms of different electro negativities. Similarly, the metallic bond
is not a covalent bond,as the metal atoms do not have sufficient number of
valence electrons for mutual sharing with 8 or 12 neighboring metal atoms in a
crystal. So, we have to search for a new theory to explain metallic bond. The
first successful theory is due to Drude and Lorentz, which regards metallic
crystal as an assemblage of positive ions immersed in a gas of free electrons.
The free electrons are due to ionization of the valence electrons of the atoms
of the metal. As the valence electrons of the atoms are freely shared by all
the ions in the crystal, the metallic bonding is also referred to as electronic
bonding. As the free electrons repel each other, they are uniformly distributed
around the metal ions. Many physical properties of the metals can be explained
by this theory, nevertheless there are exceptions.
The
electrostatic attraction between the metal ions and the free electrons yields a
three-dimensional close packed crystal with a large number of nearest metal
ions. So, metals have high density. As the close packed structure contains many
slip planes along which movement can occur during mechanical loading, the metal
acquires ductility. Pure metals can undergo 40 to 60% elongation prior to
rupturing under mechanical loading. As each metal ion is surrounded by electron
cloud in all directions, the metallic bonding has no directional properties.
As
the electrons are free to move around the positive ions, the metals exhibit
high electrical and thermal conductivity. The metallic luster is due to
reflection of light by the electron cloud. As the metallic bond is strong
enough, the metal atoms are reluctant to break apart into a liquid or gas, so
the metals have high melting and boiling points.
The
bonding in metal is better treated by Molecular orbital theory. As per this
theory, the atomic orbitals of large number of atoms in a crystal overlap to
form numerous bonding and antibonding molecular orbitals without any band gap.
The bonding molecular orbitals are completely filled with an electron pair in
each, and the antibonding molecular orbitals are empty. Absence of band gap
accounts for high electrical conductivity of metals. High thermal conductivity
is due to thermal excitation of many electrons from the valence band to the
conductance band. With an increase in temperature, the electrical conductivity
decreases due to vigorous thermal motion of lattice ions that disrupts the
uniform lattice structure, that is required for free motion of electrons within
the crystal. Most metals are black except copper, silver and gold. It is due to
absorption of light of all wavelengths. Absorption of light of all wavelengths
is due to absence of bandgap in metals.
Related Topics