Chemical bonding | Chemistry - Bond parameters | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Bond parameters
Bond
parameters
A
covalent bond is characterised by parameters such as bond length, bond angle,
bond order etc... A brief description of some of the bond parameters is given
below.
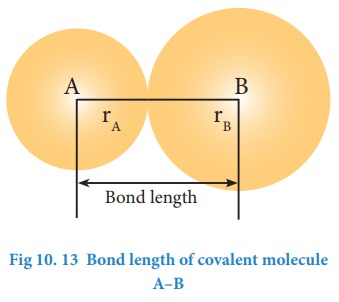
Bond length
The
distance between the nuclei of the two covalently bonded atoms is called bond
length. Consider a covalent molecule A-B. The bond length is given by the sum
of the radii of the bonded atoms (rA + rB). The length of
a bond can be determined by spectroscopic, x-ray diffraction and
electron-diffraction techniques The bond length depends on the size of the atom
and the number of bonds (multiplicity) between the combining atoms.
Greater
the size of the atom, greater will be the bond length. For example,
carbon-carbon single bond length (1.54 Å) is longer than the carbon-nitrogen
single bond length (1.43 Å).
Increase
in the number of bonds between the two atoms decreases the bond length. For
example, the carbon-carbon single bond is longer than the carbon-carbon double
bond (1.33 Å) and the carbon-carbon triple bond (1.20 Å).
![]()
![]()
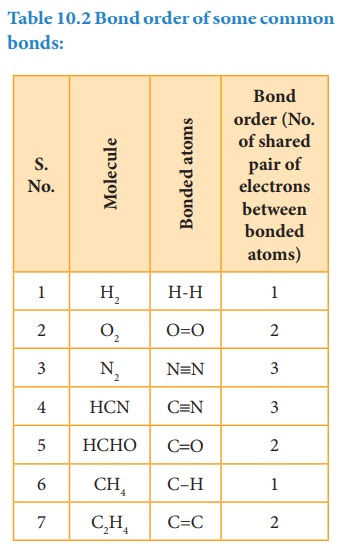
Bond order
The
number of bonds formed between the two bonded atoms in a molecule is called the
bond order. In Lewis theory, the bond order is equal to the number of shared
pair of electrons between the two bonded atoms. For example in hydrogen
molecules, there is only one shared pair of electrons and hence, the bond order
is one. Similarly, in H2O, HCl, Methane, etc the central atom forms
single bonds with bond order of one.
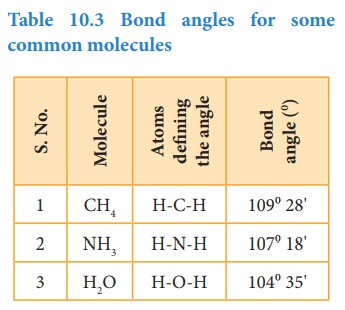
Bond angle
Covalent
bonds are directional in nature and are oriented in specific directions in
space. This directional nature creates a fixed angle between two covalent bonds
in a molecule and this angle is termed as bond angle. It is usually expressed
in degrees. The bond angle can be determined by spectroscopic methods and it
can give some idea about the shape of the molecule.
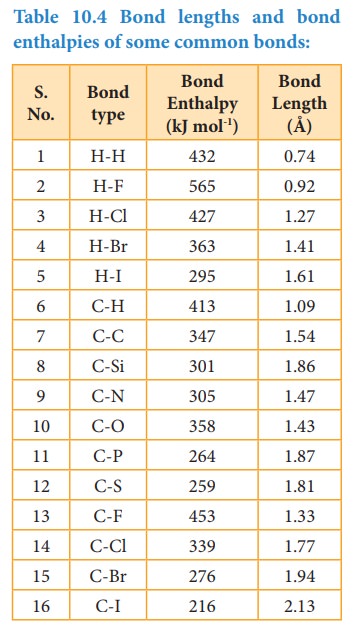
Bond enthalpy
The
bond enthalpy is defined as the minimum amount of energy required to break one
mole of a particular bond in molecules in their gaseous state. The unit of bond
enthalpy is kJ mol-1. Larger the bond enthalpy, stronger will be the
bond. The bond energy value depends on the size of the atoms and the number of
bonds between the bonded atoms. Larger the size of the atom involved in the
bond, lesser is the bond enthalpy.
In
case of polyatomic molecules with, two or more same bond types, in the term
average bond enthalpy is used. For such bonds, the arithmetic mean of the bond
energy values of the same type of bonds is considered as average bond enthalpy.
For example in water, there are two OH bonds present and the energy needed to
break them are not same.
![]()
![]() H2O(g)→H(g)+OH(g) ΔH1 = 502 kJ
mol-1
H2O(g)→H(g)+OH(g) ΔH1 = 502 kJ
mol-1
OH(g)→H(g)+O(g) ΔH2 = 427 kJ mol-1
The
average bond enthalpy of OH bond in water = 502+427 / 2 = 464.5 kJ mol-1
Resonance
When
we write Lewis structures for a molecule, more than one valid Lewis structures
are possible in certain cases. For example let us consider the Lewis structure
of carbonate ion [CO3]2-.
The
skeletal structure of carbonate ion (The oxygen atoms are denoted as OA,
OB & OC
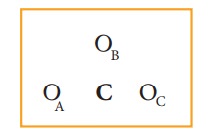
Total
number of valence electrons = [1 x 4(carbon)] + [3 x 6 (oxygen)] + [2 (charge)]
= 24 electrons.
Distribution
of these valence electrons gives us the following structure.
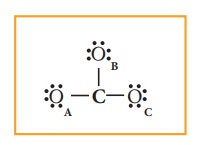
Complete
the octet for carbon by moving a lone pair from one of the oxygens (OA)
and write the charge of the ion (2-) on the upper right side as shown in the
figure.
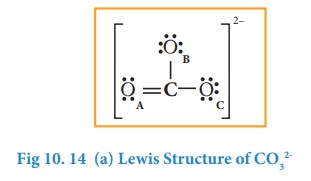
In
this case, we can draw two additional Lewis structures by moving the lone pairs
from the other two oxygens (OB and OC) thus creating
three similar structures as shown below in which the relative position of the
atoms are same. They only differ in the position of bonding and lone pair of
electrons. Such structures are called resonance structures (canonical
structures) and this phenomenon is called resonance.
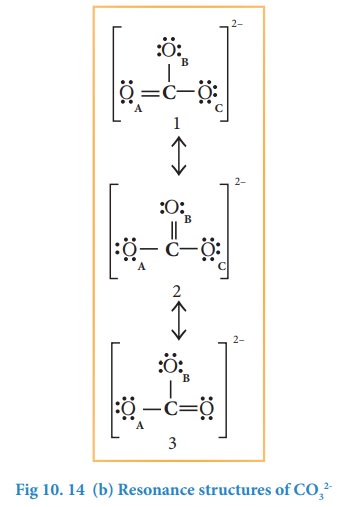
It
is evident from the experimental results that all carbon-oxygen bonds in
carbonate ion are equivalent. The actual structure of the molecules is said to
be the resonance hybrid, an average of these three resonance forms. It is
important to note that carbonate ion does not change from one structure to
another and vice versa. It is not possible to picturise the resonance hybrid by
drawing a single Lewis structure. However, the following structure gives a
qualitative idea about the correct structure.
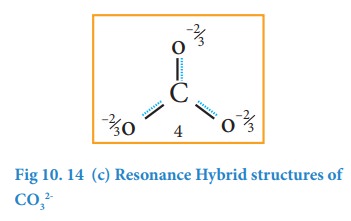
It
is found that the energy of the resonance hybrid (structure 4) is lower than
that of all possible canonical structures (Structure 1, 2 & 3). The
difference in energy between structure 1 or 2 or 3, (most stable canonical
structure) and structure 4 (resonance hybrid) is called resonance energy.
Polarity of Bonds
Partial ionic character in covalent bond:
When
a covalent bond is formed between two identical atoms (as in the case of H2,
O2, Cl2 etc...) both atoms have equal tendency to attract
the shared pair of electrons and hence the shared pair of electrons lies
exactly in the middle of the nuclei of two atoms. However, in the case of
covalent bond formed between atoms having different electronegativities, the
atom with higher electronegativity will have greater tendency to attract the
shared pair of electrons more towards itself than the other atom. As a result
the cloud of shared electron pair gets distorted.
Let
us consider the covalent bond between hydrogen and fluorine in hydrogen fluoride.
The electronegativities of hydrogen and fluorine on Pauling's scale are 2.1 and
4 respectively. It means that fluorine attracts the shared pair of electrons
approximately twice as much as the hydrogen which leads to partial negative
charge on fluorine and partial positive charge on hydrogen. Hence, the H-F bond
is said to be polar covalent bond.
![]()
![]() Here, a very small, equal and opposite charges are separated
by a small distance (91 pm) and is referred to as a dipole.
Here, a very small, equal and opposite charges are separated
by a small distance (91 pm) and is referred to as a dipole.
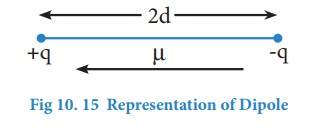
Dipole moment:
The
polarity of a covalent bond can be measured in terms of dipole moment which is
defined as
μ
= q × 2d
Where
μ is the dipole moment, q is the charge and 2d is the distance between the two
charges. The dipole moment is a vector and the direction of the dipole moment
vector points from the negative charge to positive charge.
The
unit for dipole moment is columb meter (C m). It is usually expressed in Debye
unit (D). The conversion factor is 1 Debye = 3.336 x 10-30 C m
Diatomic
molecules such as H2, O2, F2 etc... have zero
dipole moment and are called non polar molecules and molecules such as HF, HCl,
CO, NO etc... have non zero dipole moments and are called polar molecules.
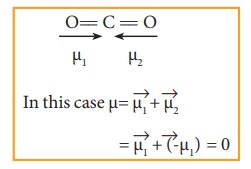
Molecules
having polar bonds will not necessarily have a dipole moment. For example, the
linear form of carbon dioxide has zero dipole moment, even though it has two
polar bonds. In CO2, the dipole moments of two polar bonds (CO) are
equal in magnitude but have opposite direction. Hence, the net dipole moment of
the CO2 is, μ = μ1 + μ2 = μ1 + (-μ1)
= 0
Incase
of water net dipole moment is the vector sum of μ1+ μ2 as
shown.
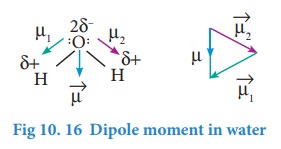
Dipole
moment in water is found to be 1.85D
Table 10. 5 Dipole
moments of common molecules
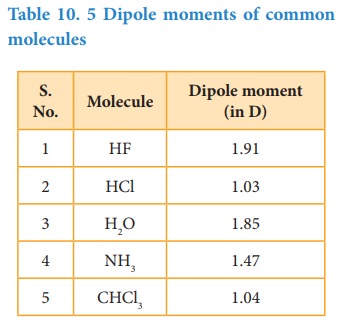
The
extent of ionic character in a covalent bond can be related to the electro
negativity difference to the bonded atoms. In a typical polar molecule,
Aδ--Bδ+, the electronegativity difference (χA- χB) can be
used to predict the percentage of ionic character as follows.
![]()
![]() If the electronegativity difference (χA- χB
), is
If the electronegativity difference (χA- χB
), is
equal
to 1.7, then the bond A-B has 50% ionic character
if
it is greater than 1.7, then the bond A-B has more than 50% ionic character, and
if it is lesser than 1.7, then the bond A-B has less than 50% ionic character.
Partial covalent character in ionic bonds:
Like
the partial ionic character in covalent compounds, ionic compounds show partial
covalent character. For example, the ionic compound, lithium chloride shows
covalent character and is soluble in organic solvents such as ethanol.
The partial covalent character in ionic compounds can be explained on the basis of a phenomenon called polarisation. We know that in an ionic compound, there is an electrostatic attractive force between the cation and anion. The positively charged cation attracts the valence electrons of anion while repelling the nucleus. This causes a distortion in the electron cloud of the anion and its electron density drifts towards the cation, which results in some sharing of the valence electrons between these ions. Thus, a partial covalent character is developed between them. This phenomenon is called polarisation.
The
ability of a cation to polarise an anion is called its polarising ability and
the tendency of an anion to get polarised is called its polarisability. The
extent of polarisation in an ionic compound is given by the Fajans rules
Fajans Rules
(i)
To show greater covalent character, both the cation and anion should have high
charge on them. Higher the positive charge on the cation, greater will be the
attraction on the electron cloud of the anion. Similarly higher the magnitude
of negative charge on the anion, greater is its polarisability. Hence, the
increase in charge on cation or in anion increases the covalent character
Let
us consider three ionic compounds aluminum chloride, magnesium chloride and
sodium chloride. Since the charge of the cation increase in the order Na+
< Mg2+ < Al3+, the covalent character also follows
the same order NaCl < MgCl2 < AlCl3.
(ii)
The smaller cation and larger anion show greater covalent character due to the
greater extent of polarisation.
Lithium
chloride is more covalent than sodium chloride. The size of Li+ is
smaller than Na+ and hence the polarising power of Li+ is
more. Lithium iodide is more covalent than lithium chloride as the size of I-
is larger than the Cl-. Hence I- will be more polarised
than Cl- by the cation, Li+ .
(iii)
Cations having ns2 np6 nd10 configuration show
greater polarising power than the cations with ns2 np6 configuration. Hence,
they show greater covalent character.
CuCl
is more covalent than NaCl. Compared to Na+ (1.13 Å) . Cu+ (0.6 Å)
is small and have 3s2 3p6 3d10 configuration.
Electronic
configuration of Cu+ [Ar] 3s2, 3p6, 3d10
![]()
![]() Electronic Configuration of Na+ [He] 2s2,
2p6
Electronic Configuration of Na+ [He] 2s2,
2p6
Valence Shell Electron Pair Repulsion (VSEPR) theory
Lewis
concept of structure of molecules deals with the relative position of atoms in
the molecules and sharing of electron pairs between them. However, we cannot
predict the shape of the molecule using Lewis concept. Lewis theory in
combination with VSEPR theory will be useful in predicting the shape of
molecules.
Important principles of VSEPR Theory are as follows:
1.
The shape of the molecules depends on the number of valence shell electron pair
around the central atom.
2.
There are two types of electron pairs namely bond pairs and lone pairs. The
bond pair of electrons are those shared between two atoms, while the lone pairs
are the valence electron pairs that are not involved in bonding.
3.
Each pair of valence electrons around the central atom repels each other and
hence, they are located as far away as possible in three dimensional space to
minimize the repulsion between them.
4.
The repulsive interaction between the different types of electron pairs is in
the following order.
lp - lp > lp - bp> bp-bp
lp- lone pair ; bp- bond pair
The
lone pair of electrons are localised only on the central atom and interacts
with only one nucleus whereas the bond pairs are shared between two atoms and
they interact with two nuclei. Because of this the lone pairs occupy more space
and have greater repulsive power than the bond pairs in a molecule.
The
following Table illustrates the shapes of molecules predicted by VSEPR theory.
Consider a molecule ABx where A is the central atom and x represents
the number of atoms of B covalently bonded to the central atom A. The lone
pairs present in the atoms are denoted as L.
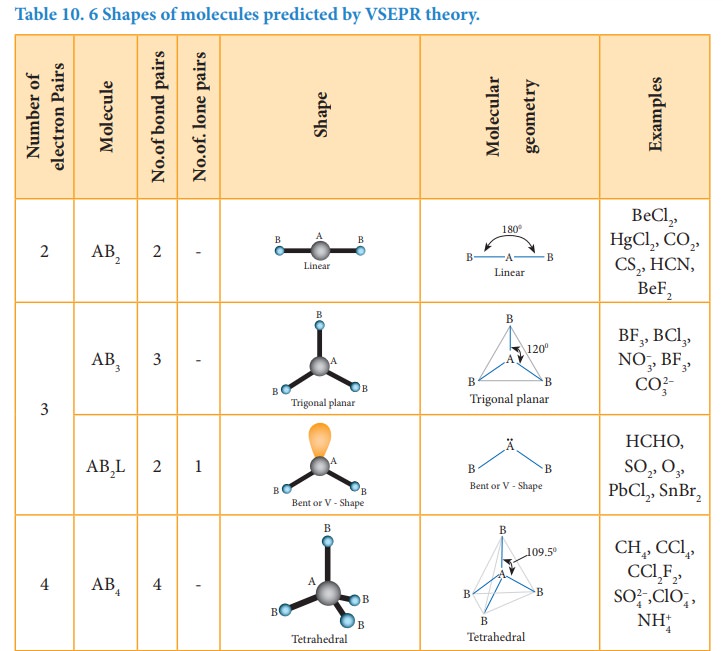
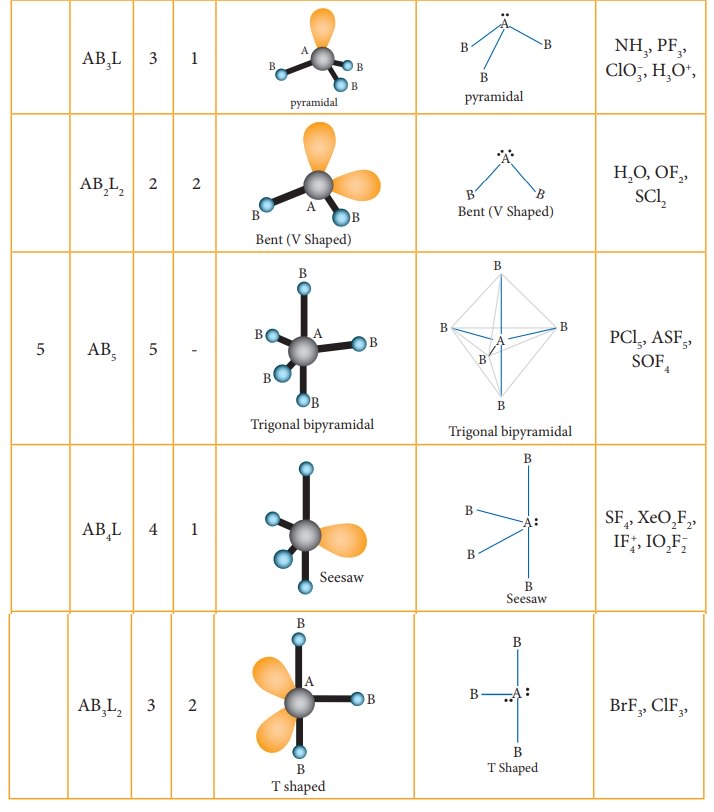
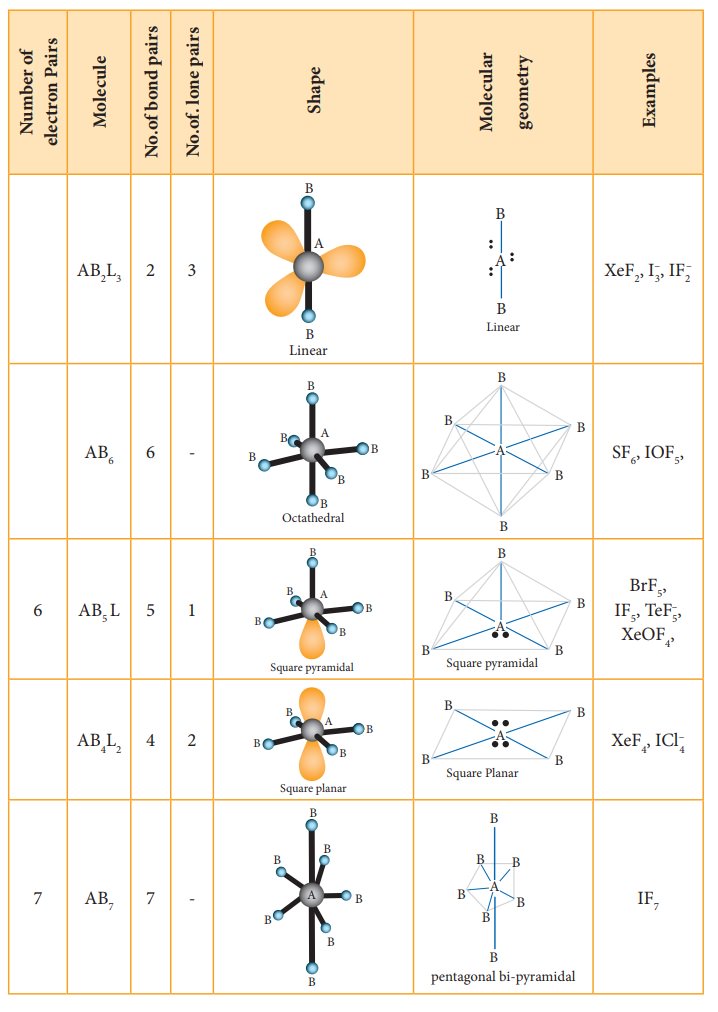
Related Topics