Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Lewis structures for exceptions to octet rule
Lewis structures for exceptions to octet rule
The octet rule is useful for writing Lewis structures for molecules with second period element as central atoms. In some molecules, the central atoms have less than eight electrons around them while some others have more than eight electrons. Exception to the octet rule can be categorized into following three types.
1. Molecules with electron deficient central atoms
2. Molecules containing odd electrons
3. Molecules with expanded valence shells
1. Molecules with electron deficient central atoms
Let us consider boron trifluoride, as an example. The central atom boron has three valence electron and each fluorine has seven valence electrons. The Lewis structure is
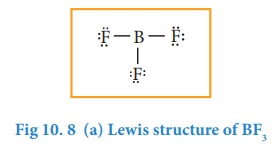
In the above structure, only six electrons around boron atom. Moving a lone pair from one of the fluorine to form additional bond as shown below.
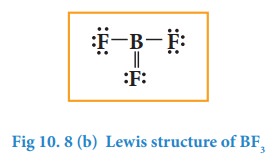
However, the above structure is unfavourable as the most electronegative atom fluorine shows positive formal charge and hence the structure with incomplete octet is the favourable one. Molecules such as BCl3, BeCl2, etc... also have incomplete octets.
2. Molecules containing odd electrons
Few molecules have a central atom with an odd number of valence electrons. For example, in nitrogen dioxide and nitric oxide all the atoms does not have octet configuration. The lewis structure of the above molecules are shown in the figure.
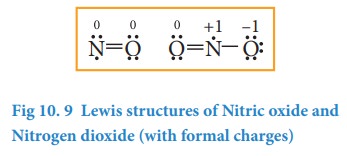
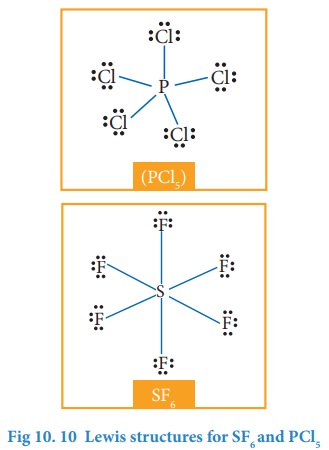
3. Molecules with expanded valence shells
In molecules such as sulphur hexafluoride (SF 6), phosphorous pentachloride (PCl5) the central atom has more than eight valence electrons around them. Here the central atom can accommodate additional electron pairs by using outer vacant d orbitals. In SF6 the central atom sulphur is surrounded by six bonding pair of electrons or twelve electrons.
Related Topics