Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Resonance - Chemical bonding
Resonance
When we write Lewis structures for a molecule, more than one valid Lewis structures are possible in certain cases. For example let us consider the Lewis structure of carbonate ion [CO3]2-.
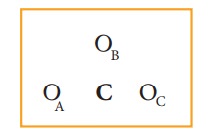
The skeletal structure of carbonate ion (The oxygen atoms are denoted as OA, OB & OC
Total number of valence electrons = [1 x 4(carbon)] + [3 x 6 (oxygen)] + [2 (charge)] = 24 electrons.
Distribution of these valence electrons gives us the following structure.
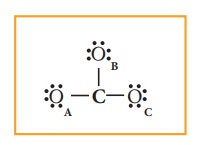
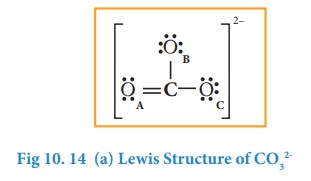
Complete the octet for carbon by moving a lone pair from one of the oxygens (OA) and write the charge of the ion (2-) on the upper right side as shown in the figure.
In this case, we can draw two additional Lewis structures by moving the lone pairs from the other two oxygens (OB and OC) thus creating three similar structures as shown below in which the relative position of the atoms are same. They only differ in the position of bonding and lone pair of electrons. Such structures are called resonance structures (canonical structures) and this phenomenon is called resonance.
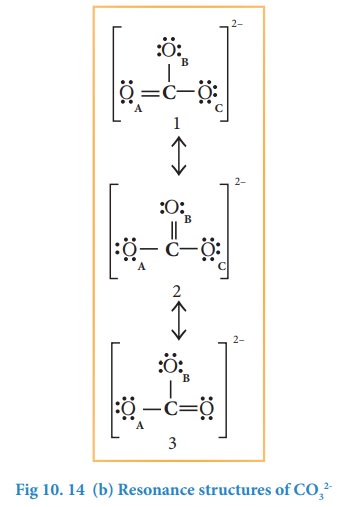
It is evident from the experimental results that all carbon-oxygen bonds in carbonate ion are equivalent. The actual structure of the molecules is said to be the resonance hybrid, an average of these three resonance forms. It is important to note that carbonate ion does not change from one structure to another and vice versa. It is not possible to picturise the resonance hybrid by drawing a single Lewis structure. However, the following structure gives a qualitative idea about the correct structure.
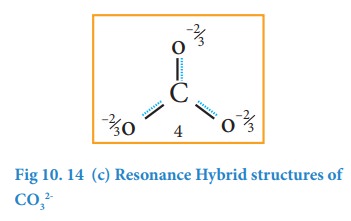
It is found that the energy of the resonance hybrid (structure 4) is lower than that of all possible canonical structures (Structure 1, 2 & 3). The difference in energy between structure 1 or 2 or 3, (most stable canonical structure) and structure 4 (resonance hybrid) is called resonance energy.
Related Topics