Chemical bonding - Types of hybridisation and geometry of molecules | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Types of hybridisation and geometry of molecules
Hybridisation
Bonding
in simple molecules such as hydrogen and fluorine can easily be explained on
the basis of overlap of the respective atomic orbitals of the combining atoms.
But the observed properties of polyatomic molecules such as methane, ammonia,
beryllium chloride etc... cannot be explained on the basis of simple overlap of
atomic orbitals. For example, it was experimentally proved that methane has a
tetrahedral structure and the four C-H bonds are equivalent. This fact cannot
be explained on the basis of overlap of atomic orbitals of hydrogen (1s) and
the atomic orbitals of carbon with different energies (2s2 2px2
2py 2pz).
In
order to explain these observed facts, Linus Pauling proposed that the valence
atomic orbitals in the molecules are different from those in isolated atom and
he introduced the concept of hybridisation. Hybridisation is the process of
mixing of atomic orbitals of the same atom with comparable energy to form equal
number of new equivalent orbitals with same energy. The resultant orbitals are
called hybridised orbitals and they posses maximum symmetry and definite
orientation in space so as to minimize the force of repulsion between their
electrons .
Types
of hybridisation and geometry of molecules
sp Hybridisation:
Let
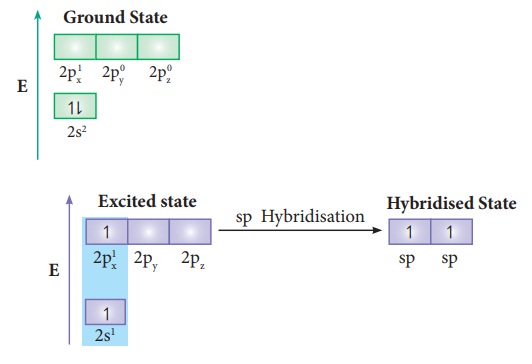
us consider the bond formation in beryllium chloride. The valence shell
electronic configuration of beryllium in the ground state is shown in the
figure.
In
BeCl2 both the Be-Cl bonds are equivalent and it was observed that
the molecule is linear. VB theory explain this observed behaviour by sp
hybridisation. One of the paired electrons in the 2s orbital gets excited to 2p
orbital and the electronic configuration at the excited state is shown.
![]()
![]() Now, the 2s and 2p orbitals hybridise and produce two
equivalent sp hybridised orbitals which have 50 % s-character and 50 %
p-character. These sp hybridised orbitals are oriented in opposite direction as
shown in the figure.
Now, the 2s and 2p orbitals hybridise and produce two
equivalent sp hybridised orbitals which have 50 % s-character and 50 %
p-character. These sp hybridised orbitals are oriented in opposite direction as
shown in the figure.
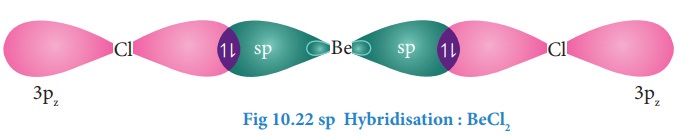
Overlap with orbital of chlorine
Each
of the sp hybridized orbitals linearly overlap with pz orbital of
the chlorine to form a covalent bond between Be and Cl as shown in the Figure.
sp2 Hybridisation:
Consider
boron trifluoride molecule. The valence shell electronic configuration of boron
atom is [He]2s22p1.
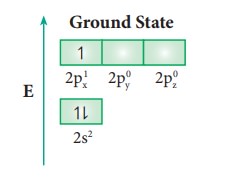
In
the ground state boron has only one unpaired electron in the valence shell. In
order to form three covalent bonds with fluorine atoms, three unpaired
electrons are required. To achieve this, one of the paired electrons in the 2s
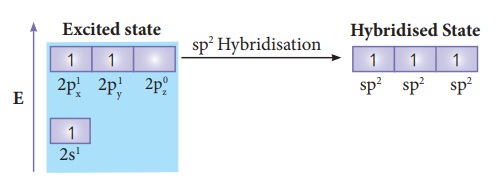
orbital is promoted to the 2py orbital in the excite state.
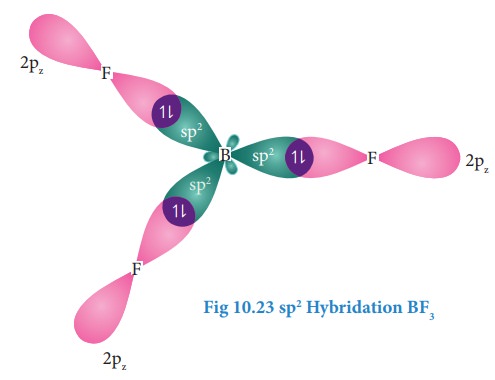
In boron, the s orbital and two p orbitals (px and py) in the valence shell hybridses, to generate three equivalent sp2 orbitals as shown in the Figure. These three orbitals lie in the same xy plane and the angle between any two orbitals is equal to 120º
Overlap with 2pz orbitals of fluorine:
The
three sp2 hybridised orbitals of boron now overlap with the 2pz
orbitals of fluorine (3 atoms). This overlap takes place along the axis as
shown below.
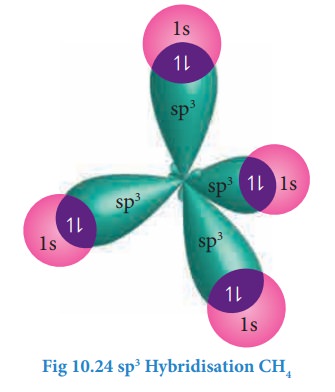
sp3 Hybridisation:
sp3
hybridisation can be explained by considering methane as an example. In methane
molecule the central carbon atom bound to four hydrogen atoms. The ground state
valence shell electronic configuration of carbon is [He]2s2 2px1
2py1 2pz0.
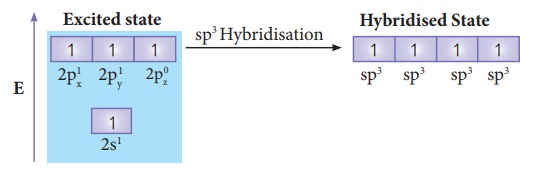
In
order to form four covalent bonds with the four hydrogen atoms, one of the
paired electrons in the 2s orbital of carbon is promoted to its 2pz
orbital in the excite state. The one 2s orbital and the three 2p orbitals of
carbon mixes to give four equivalent sp3 hybridised orbitals. The angle between
any two sp3 hybridised orbitals is 109⁰ 28'
![]()
![]()
Overlap with 1s orbitals of hydrogen:
sp3d Hybridisation:
In
the molecules such as PCl5, the central
atom phosphorus is
covalently bound to five chlorine atoms. Here the atomic orbitals of
phosphorous undergoes sp3d hybridisation which involves its one 3s
orbital, three 3p orbitals and one vacant 3d orbital (dz2). The
ground state electronic configuration of phosphorous is [Ne]3s2 3p1x3py13pz1
as shown below.
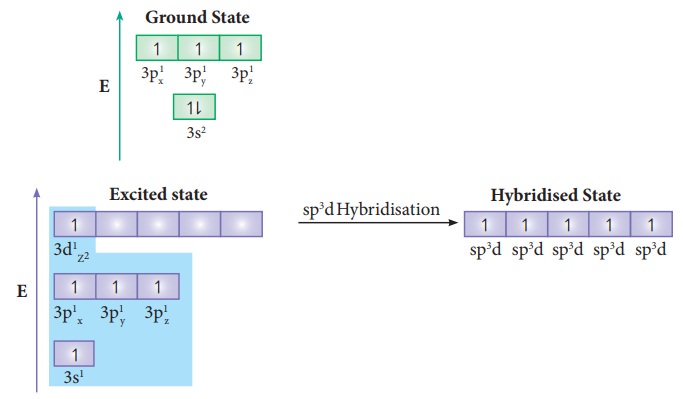
One
of the paired electrons in the 3s orbital of phosphorous is promoted to one of its
vacant 3d orbital (dz2) in the excite state. One 3s
orbital, three 3p orbitals and one 3dz2 orbital of
phosphorus atom mixes to give five equivalent sp3d hybridised orbitals. The orbital
geometry of sp3d hybridised orbitals is trigonal
bi-pyramidal as shown in the figure 10. x.
Overlap with 3pz orbitals of chlorine:
The
3pz orbitals of the five chlorine atoms linearly overlap along the
axis with the five sp3d hybridised orbitals of phosphorous to form
the five P-Cl σ-bonds, as shown below.
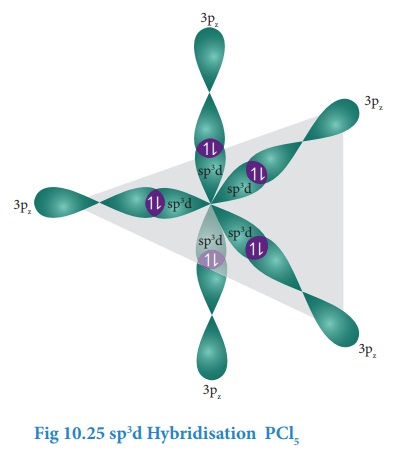
sp3d2 Hybridisation:
In
sulphur hexafluoride (SF6) the central atom sulphur extend its octet
to undergo sp3d2 hybridisation to generate six sp3d2 hybridised orbitals which accounts for six
equivalent S-F bonds. The ground state electronic configuration of sulphur
is[Ne]3s2 3px1 3py1 3pz1.
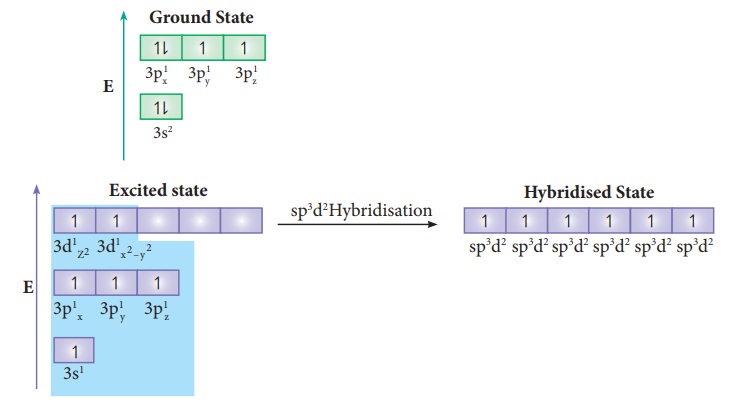
One electron each from 3s orbital and 3p orbital of sulphur is promoted to its two vacant 3d orbitals (dz2 and dx2-y2) in the excite state. A total of six valence orbitals from sulphur (one 3s orbital, three 3p orbitals and two 3d orbitals) mixes to give six equivalent sp3d2 hybridised orbitals. The orbital geometry is octahedral as shown in the figure.
Overlap with 2pz orbitals of fluorine:
The
six sp3d2
hybridised orbitals of sulphur overlaps linearly with 2pz orbitals of six fluorine atoms to
form the six S-F bonds in the sulphur hexafluoride molecule.
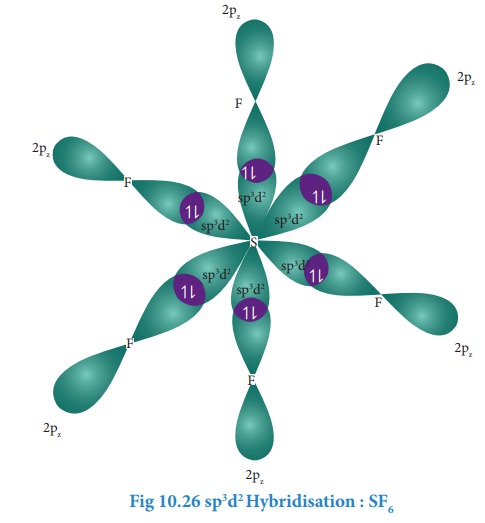
Bonding in ethylene:
The
bonding in ethylene can be explained using hybridisation concept. The molecular
formula of ethylene is C2H4. The valency of carbon is 4.
The electronic configuration of valence shell of carbon in ground state is
[He]2s2 2px1 2py1 2pz0.
To satisfy the valency of carbon promote an electron from 2s orbital to 2pz
orbital in the excited state.
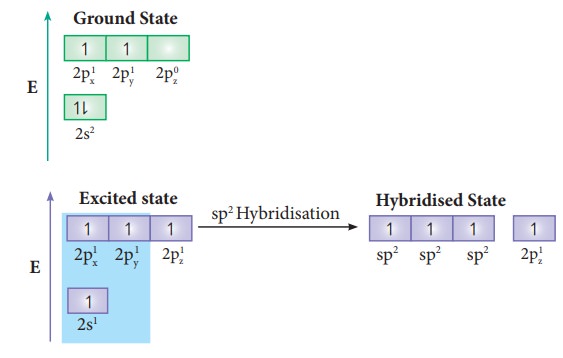
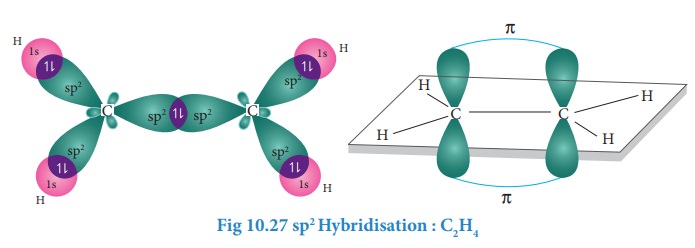
In ethylene both the carbon atoms undergoes sp2 hybridisation involving 2s, 2px and 2py orbitals, resulting in three equivalent sp2 hybridised orbitals lying in the xy plane at an angle of 120⁰ to each other. The unhybridised 2pz orbital lies perpendicular to the xy plane.
Formation of sigma
bond:
One
of the sp2 hybridised orbitals of each carbon lying on the molecular
axis (x-axis) linearly overlaps with each other resulting in the formation a
C-C sigma bond. Other two sp2 hybridised orbitals of both carbons linearly
overlap with the four 1s orbitals of four hydrogen atoms leading to the
formation of two C-H sigma bonds on each carbon.
Formation of pi bond:
The
unhybridised 2pz orbital of both carbon atoms can overlap only
sideways as they are not in the molecular axis. This lateral overlap results in
the formation a pi bond between the two carbon atoms as shown in the figure.
Bonding in acetylene:
Similar
to ethylene, the bonding in acetylene can also be explained using hybridization
concept. The molecular formula of acetylene is C2H2. The
electronic configuration of valence shell of carbon in ground state is [He]2s2
2p1x 2p1y 2p0z.
To satisfy the valency of carbon promote an electron from 2s orbital to 2pz orbital in the excited
state.
In acetylene molecule, both the carbon atoms are in sp hybridised state. The 2s and 2px orbitals, resulting in two equivalent sp hybridised orbitals lying in a straight line along the molecular axis (x-axis). The unhybridised 2py and 2pz orbitals lie perpendicular to the ymolecularz axis.
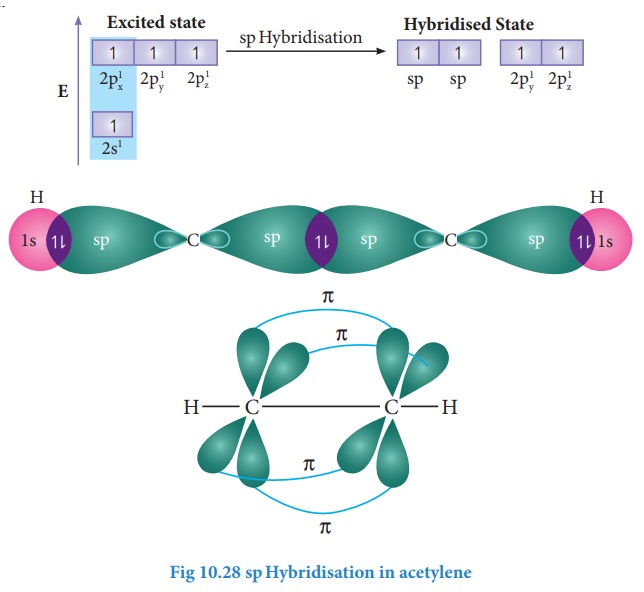
Formation of sigma bond:
One of the two sp hybridised orbitals of each carbon linearly overlaps with each other resulting in the formation a C-C sigma bond. The other sp hybridised orbital of both carbons linearly overlap with the two 1s orbitals of two hydrogen atoms leading to the formation of one C-H sigma bonds on each carbon.
Formation of pi bond:
The
unhybridised 2py and 2pz orbitals of each carbon overlap
sideways. This lateral overlap results in the formation of two pi bonds ( py-py and pz-pz) between the
two carbon atoms as shown in the figure.
Related Topics