Chemical bonding - Bond enthalpy | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Bond enthalpy
Bond enthalpy
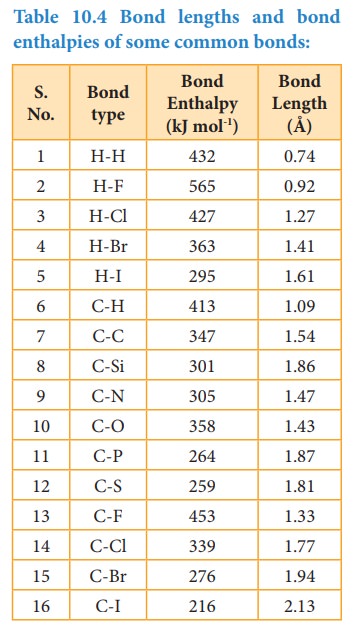
The bond enthalpy is defined as the minimum amount of energy required to break one mole of a particular bond in molecules in their gaseous state. The unit of bond enthalpy is kJ mol-1. Larger the bond enthalpy, stronger will be the bond. The bond energy value depends on the size of the atoms and the number of bonds between the bonded atoms. Larger the size of the atom involved in the bond, lesser is the bond enthalpy.
In case of polyatomic molecules with, two or more same bond types, in the term average bond enthalpy is used. For such bonds, the arithmetic mean of the bond energy values of the same type of bonds is considered as average bond enthalpy. For example in water, there are two OH bonds present and the energy needed to break them are not same.
![]()
![]() H2O(g)→H(g)+OH(g) ΔH1 = 502 kJ mol-1
H2O(g)→H(g)+OH(g) ΔH1 = 502 kJ mol-1
OH(g)→H(g)+O(g) ΔH2 = 427 kJ mol-1
The average bond enthalpy of OH bond in water = 502+427 / 2 = 464.5 kJ mol-1
Related Topics