Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Brief questions and answers: Chemistry: Chemical bonding
Chemical bonding | Chemistry
Answer the following questions
31. Define the following
i. Bond order
ii. Hybridisation
iii. σ- bond
Bond order :
Bond
order gives the number of covalent bonds berween the two combining atoms
Bond
order = [ Nb – Na ] / 2
Hybridisation
:
Hybridisation
is the process of mixing of atomic orbitals of the same atom with comparable
energy to form equal number of new equivalent orbitals with same energy.
σ - bend :
When
two atomic orbitals overlap linearly along the axis, the resultant bond is
called a sigma (σ) bond. This overlap is also called ‘head -on everlap’ or
‘axial overlap’.
32. What is a pi bond?
When
two atomic orbitals overlaps sideways, the resultant covalent bond is called a
pi (π) bond.
33. In CH4, NH3 and H2O, the central atom undergoes sp3 hybridisation - yet their bond angles are different. why?
i)
Methane 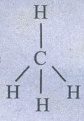 Tetrahedral (AB4 type)
Tetrahedral (AB4 type)
No
lone pair of electrons
Hence,
bond angle is 109°28' (or) 109.5°
ii)
Ammonia 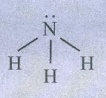 pyramidal shape (AB3L type)
pyramidal shape (AB3L type)
Number
of lone pairs = 1
Due
to lp - bp repulsion, bond angle decreases to 107° 18’
iii)
water 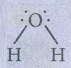 Bent (V-shaped) - AB2L2 type.
Bent (V-shaped) - AB2L2 type.
Number
of lone pairs = 2
Due
to lp-bp repulsion, bond angle decreases to 104° 35’
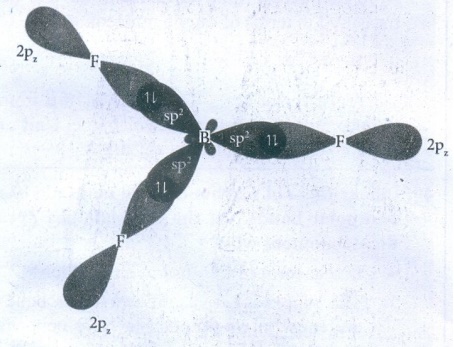
34. Explain Sp2 hybridisation in BF3
The
electronic configuration of Boron
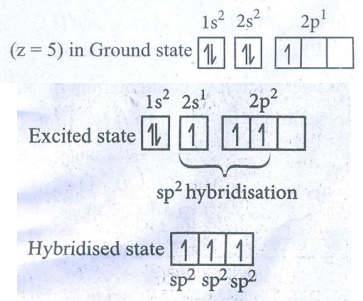
●
In the GS, Boron has only one unpaired electron in the valence shell.
●
Three unpaired e− s are required to form three covalent bonds with
fluorine atoms.
●
So, one electron from the 2s orbital is promoted to the 2p orbital in the
excited state.
●
Hence, three equivalent sp2 hybridised orbitals are generated.
●
This three sp2 hybridised orbitals of Boron now overlap with the 2p
orbitals of three Fluorine atoms.
●
This overlap takes place along the axis and forming three covalent bonds.
35. Draw the M.O diagram for oxygen molecule calculate its bond order and show that O2 is paramagnetic.
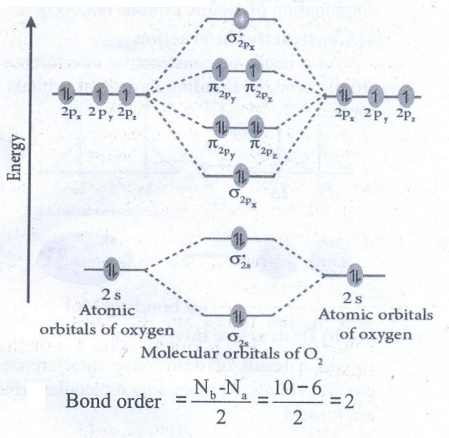
●
Bond order = [ Nb – Na ] / 2 = [10 – 6] / 2 = 2
●
Oxygen molecule has two unpaired electrons. Hence, it is paramagnetic.
36. Draw MO diagram of CO and calculate its bond order.
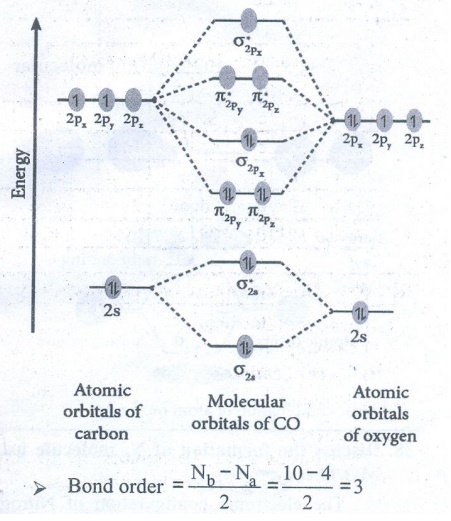
●
Bond order = [ Nb – Na ] / 2 = [10–4] / 2 = 3
37. What do you understand by Linear combination of atomic orbitals in MO theory.
There
are two interactions of Linear combination of atomic orbitals (LCAO)
a)
Constructive interaction
As
a result of constructive interference of atomic orbitals, Bonding molecular
orbitals are formed.
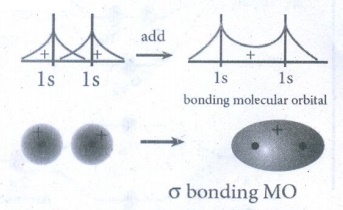
b)
Destructive interaction
As
a result of destructive interference of atomic orbitals, Anti bonding molecular
orbitals are formed.
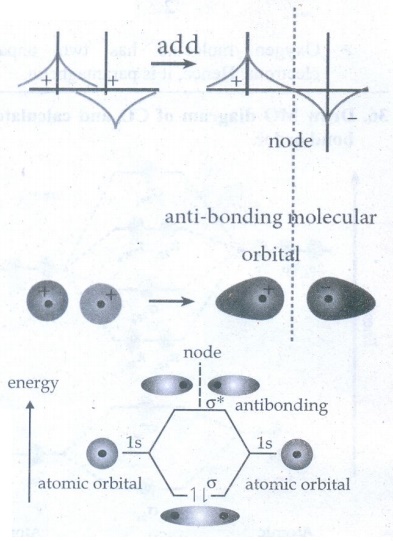
Linear combination of atom molecules
38. Discuss the formation of N2 molecule using MO Theory
The
electronic configuration of Nitrogen atom 1s2 2s2 sp3
The
electronic configuration of Nitrogen molecule (σ1s )2 (σ*1s
)2 (σ2s )2 (σ*2s )2 (π2py
)2 (π2pz )2 (σ2px )2
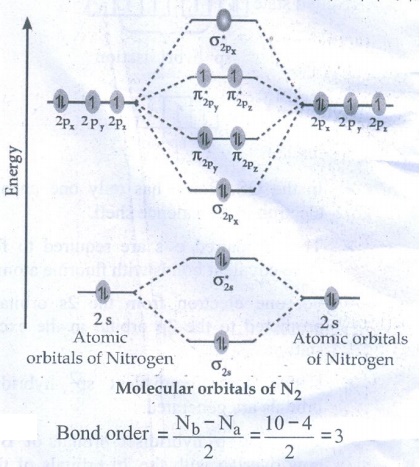
●
Bond order = [ Nb – Na ] / 2 = [10 – 4] / 2 = 3
●
N2 molecule has no unpaired electrons. Hence, it is diamagnetic.
39. What is dipolment?
The
polarity of a covalent bond can be measured in terms of dipole moment.
μ = q × 2d
μ
is a vector scale. The direction of μ from the negative charge to positive
charge. Unit of Dipolement is columb meter (Cm).
40. Linear form of carbondioxide molecule has two polar bonds. yet the molecule has Zero dipolement why?
●
Linear form of CO2 has two polar bonds.
●
The dipole moments of two polar bonds are equal in magnitude but have opposite
direction.
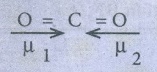
●
The net dipole moment of CO2 is = 0
That is, 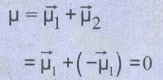
41. Draw the Lewis structures for the following species.
i) NO3– ii) SO42– iii) HNO3 iv) O3
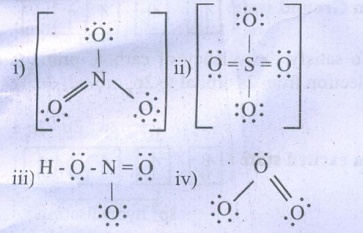
42. Explain the bond formation in BeCl2 and MgCl2.
Formation
of BeCl2:
Electronic
configuration of Be (Z = 4) in
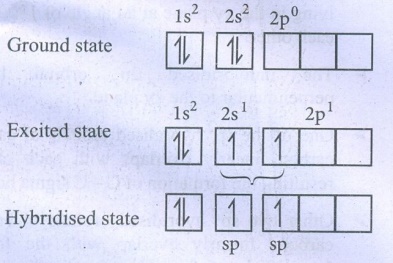
●
Each of the sp hybridised orbitals linearly overlap with orbital of the
chlorine to form a covalent bond between Be and Cl.
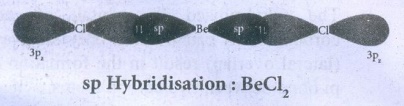
Formation of MgCl2
●
Mg (z = 12) loses two e−s and forming a stable configuration of Ne.
Mg
→ Mg2+ + 2e-
(2,8,2)
(2,8)
●
Two Cl(z = 17) accepts two e−s (one per each) and forming a stable
configuration of Ar.
2Cl + 2e− → 2Cl−
(2,8,7)
(2,8,8)
●
One Mg2+ and two Cl− ions are held together by
electrostatic attractive force.
43. Which bond is stronger σ or π? Why?
●
σ - bond is stronger than the π-bond.
●
The strength of a covalent bond depends upon the extent of overlap of atomic
orbitals.
●
Greater the overlap, larger is the energy released and stronger will be the
bond formed.
●
Overlaping along the axis (σ bond) is greater than the sidewise overlaping (π
-bond).
44. Define bond energy.
The
bond energy is defined as the minimum amount of energy required to break one
mole of a particular bond in molecule in their gaseous state. The unit of bond
energy is kJmol−1.
45. Hydrogen gas is diatomic where as inert gases are monoatomic – explain on the basis of MO theory.
●
According to MO theory,
Bond
order = [ Nb – Na ] / 2
Nb
- total number of e−s in bonding molecular orbitals.
Na
- total number of e−s in Antibonding molecular orbitals.
●
Bond order gives the number of covalent bonds between the two combining atoms.
●
From the MO diagram of H2 molecule,
Nb
= 2, Na = 0
Therefore,
Bond order = 1
H - H
Diatomic
●
From the MO diagram of Helium molecule, Nb = Na = 2
Therefore,
Bond order = 0; He2 is not formed.
So,
He like inert gases are monoatomic.
46. What is Polar Covalent bond? explain with example.
In
the case of covalent bond formed between atoms having different
electronegativities, the atom with higher electronegativity will have the
greater tendency to attract the shared pair of electrons more towards itself
than the other atom.
This
leads to partial negative charge on the atom and partial positive charge on the
another atom. This is called polar covalent bond.
Example: HF
molecule Hδ+ − F δ-
(Electronegativities
of Hydrogen and Fluorine are 2.1 and 4.0 respectively)
47. Considering x- axis as molecular axis, which out of the following will form a sigma bond.
i) 1s and 2py ii) 2Px and 2Px
iii) 2px and 2pz iv) 1s and 2pz
i)
1s and 2py form a σ-bond
Ex: H - C
bond is CH4 molecule
ii)
2PX and 2PX form a σ-bond
Ex:
B
- B bond in B2 molecule
iii)
2PX and 2PZ form a π-bond
Ex: B-F bond
in BF3 molecule
iv)
1s and 2PZ form a σ-bond
Ex: H - N
bond in NH3 molecule.
48. Explain resonance with reference to carbonate ion?
Three
possible resonance structures can be drawn for carbonate ion (CO32−).
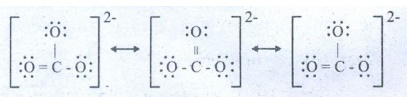
●
In this structures, the relative position of the atoms are same.
●
They only differ in the position of bonding and lone pair of electrons.
●
Such structures are called resonance structures and this phenomenon is called
resonance.
49. Explain the bond formation in ethylene and acetylene.
Bond
formation in Ethylene.
●
Molecular fomula of Ethylene is : C2H4
●
Electronic configuration of Carbon
In
Ground state : 1s2 2s2 2px1
2py1 2pz0
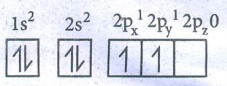
To
satisfy the valency of carbon, promote an electron from 2s orbital to 2pz
orbital.
In
excited state : 1s2 2s1 2px1
2py1 2pz1
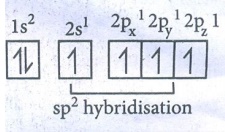
In
Hybridised state : sp2 sp2 sp2 2pz1
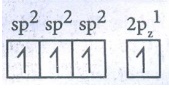
●
The three equivalent sp2 hybridised orbitals lying in the xy plane
at an angle of 1200 to each other.
●
The unhybridised 2pz orbital lies perpendicular to the xy plane.
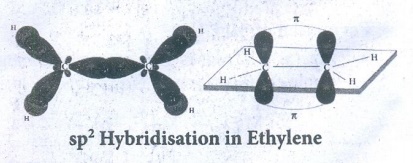
●
One of the sp2 hybridised orbitals of each carbon linearly overlaps
with each other resulting the formation of C - C sigma bond.
●
Other two sp2 hybridised orbitals of both carbons linearly everlap
with the four 1s orbitals of four H atoms leading the formation of four C - H
sigma bonds on each carbon.
●
The unhybridised 2pz orbitals of each corbon atoms can overlap only
sideways (lateral overlap) result in the formation of pi bond between two
carbon atoms.
Bond
formation in Acetylene:
●
Molecular formula of Acetylene is C2H2
●
Electronic configuration of carbon
In
ground state :1s2 2s2 2px1
2py1 2pz0
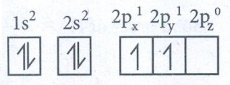
To
satisfy the valency of carbon, promote an electron from 2s orbital to 2pz
orbital.
In
excited state : 1s2 2s1 2px1
2py1 2pz1
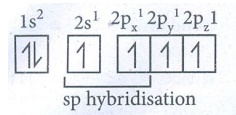
In
Hybridised state : sp sp 2py1 2pz1

●
The 2s and 2px orbitals forming two equivalent sp hybridised
orbitals.
●
The unhybridised 2py and 2pz orbitals lie perpendicular
to the molecular axis.
●
One of the two sp hybridized orbitals of each carbon linearly overlap with each
other, resulting the formation of C - C sigma bond.
●
The other sp hybridised orbital of both carbon linearly overlap with two 1s
orbitals of 2H atoms leading the formation of one C-H sigma bond on each
carbon.
●
The unhybridised 2Py and 2PZ orbitals of each Carbon
atoms can overlap sideways (lateral overlap) result in the formation of pi bond
between the two Carbon atoms.
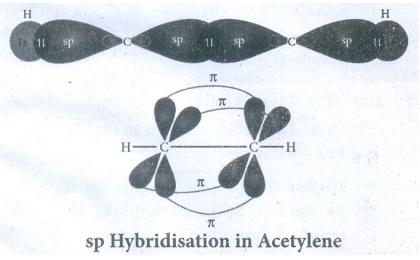
50. What type of hybridisations are possible in the following geometeries?
a) octahedral
b) tetrahedral
c) square planer.
Geometry
: Hybridisation
a)
Octahedral : sp3d2
b)
Tetrahedral : sp3
c)
Square planar : sp2d
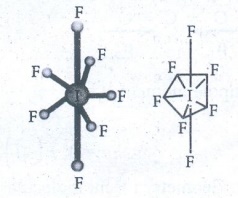
51. Explain VSEPR theory. Applying this theory to predict the shapes of IF7, and SF6
Shape
of IF7 molecule:
●
Valence shell electronic configuration of Iodine.
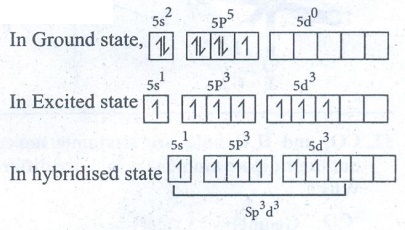
●
Iodine has 7sp3d3 hybridized orbitals.
●
This orbitals linearly overlap with 2Pz orbitals of seven F atoms,
leading the formation of 7 σ-bonds.
●
Geometry : Pentagonal bipyramid
Shape
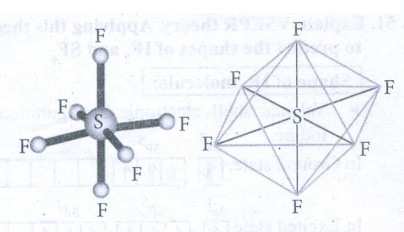
of SF6 molecule
●
Valence shell electronic configuration of
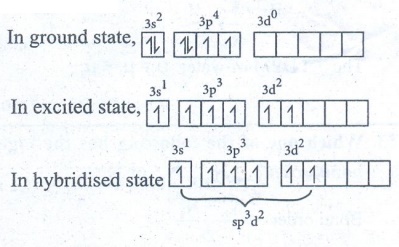
●
Sulphur has 6 sp3d2 hybridised orbitals.
●
This orbitals linearly overlap with 2Pz orbitals of six F atoms,
leading the formation of 6 σ-bonds.
●
Geometry : Octahedral.
52. CO2 and H2O both are triatomic molecule but their dipole moment values are different. Why?
CO2
Geometry : Linear
●
Eventhough CO2 has two polar bonds, Dipolemoment value is = 0
●
Reason: The DPM of two polar bonds are equal in magnitude but opposite
in direction.
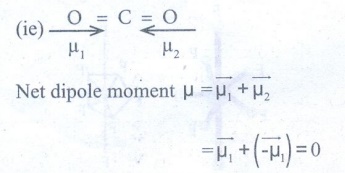
H2O
: Geometry : Bent molecule (V Shape)
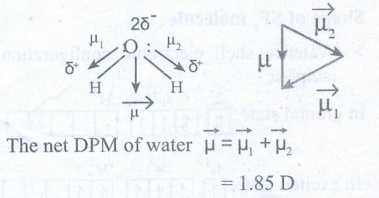
= 1.85 D
53. Which one of the following has highest bond order? N2, N+2 or N2–
Bond
order = [ Nb – Na ] / 2
Bond
order of N2 = (10 – 4) / 2 = 3
Bond
order of N2+ = (9 – 4) / 2 = 2.5
Bond
order of N2− = (10 – 5) / 2 = 2.5
Hence,
N2 molecule has the Highest bond order.
54. Explain the covalent character in ionic bond.
Ionic
compounds show partial covalent character.
Ex: LiCl is
an ionic compound. But, it shows covalent character. Therefore, it is soluble
in organic solvent like Ethanol.
This
can be explained on the basis of ‘Polarisation’.
●
The positively charged cation attracts the valence electrons of anion while
repelling the nucleus.
●
This causes a distortion in the electron cloud of the anion and its electron
density drifts towards the cation.
●
This results in some sharing of the valence electrons between these ions.
●
Thus, a partial covalent character is developed between them. This is called polarisation.
●
The ability of a cation to polarise an anion is called ‘polarising ability’.
The tendency of an anion to get polarized is called polarisability.
55. Describe fajan's rule.
The
extend of polarisation in an ionic compound is given by Fajans rule.
●
Higher the positive charge on the cation, greater will be the attraction on the
electron cloud of the cation.
●
Higher the magnitude of negative charge on the anion, greater is its
polarisability.
●
Hence, the increase in charge on cation or in anion, increases the covalent
character.
Example:
Consider
the ionic compounds: AlCl3, MgCl2 and NaCl.
Increasing
order of cationic size
Na+
< Mg2+ < Al3+
●
Order Covalent character :
Covalent
character : LiCl > NaCl
Reason: size of
Li+ < size of Na+
∴ Li+
has the higher polarising power.
●
Covalent character : Lil > LiCl
Reason:
Size
of I− > size of Cl−.
∴ I−
will be more polarised.
Related Topics