Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Linear combination of atomic orbitals
Linear combination of atomic orbitals
The wave functions for the molecular orbitals can be obtained by solving Schrödinger wave equation for the molecule. Since solving the Schrödinger equation is too complex, approximation methods are used to obtain the wave function for molecular orbitals. The most common method is the linear combination of atomic orbitals (LCAO).
We know that the atomic orbitals are represented by the wave function Ψ. Let us consider two atomic orbitals represented by the wave function ψA and ψB with comparable energy, combines to form two molecular orbitals. One is bonding molecular orbital(ψbonding) and the other is antibonding molecular orbital(ψantibonding). The wave functions for these two molecular orbitals can be obtained by the linear combination of the atomic orbitals ψA and ψB as below.
ψbonding = ψA + ψB
ψantibonding = ψA - ψB
The formation of bonding molecular orbital can be considered as the result of constructive interference of the atomic orbitals and the formation of anti-bonding molecular orbital can be the result of the destructive interference of the atomic orbitals. The formation of the two molecular orbitals from two 1s orbitals is shown below.
Constructive interaction: The two 1s orbitals are in phase and have the same sign.
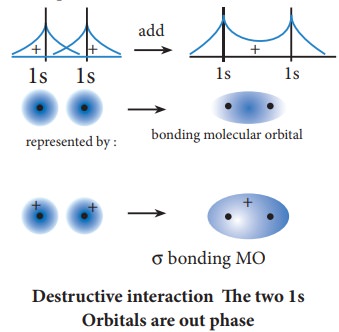
Destructive interaction The two 1s Orbitals are out phase
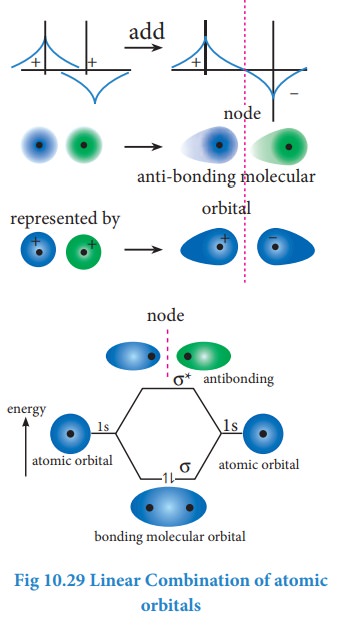
Related Topics