Multiple choice questions - Choose the best answer: Chemistry: Chemical bonding | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Choose the best answer: Chemistry: Chemical bonding
Chemistry: Chemical bonding
Evaluation
Choose the best answer
1. In which of the following Compounds does the central atom obey the octet rule?
a) XeF4
b) AlCl3
c) SF6
d) SCl2
Solution:
Compound : No. of valance electron on the central atom
XeF2 : 10
AlCl3 : 6
SF6 : 12
SCl2 : 8
2. In the molecule OA == C==OB, the formal charge on OA, C and OB are respectively.
a) -1, 0, + 1
b) +1, 0,-1
c) -2,0,+2
d) 0,0,0
Solution:
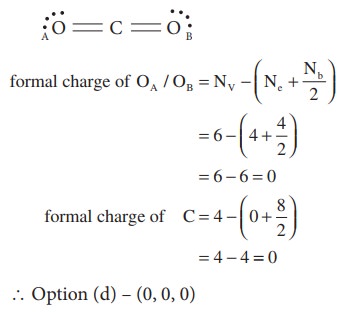
3. Which of the following is electron deficient?
a) PH3
b) (CH3)2
c) BH3
d) NH3
Solution:
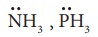 - electron rich,
- electron rich,
CH3 – CH3 – Covalent neutral molecule.
BH3 - electron deficient
4. Which of the following molecule contain no л bond?
a) SO2
b) NO2
c) CO2
d) H2O
Solution:
water contain only σ bonds and no π bonds option
5. The ratio of number of sigma (σ) and pi (л) bonds in 2- butynal is
a) 8/3
b) 5/3
c) 8/2
d) 9/2
Solution:
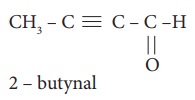
2-butynal
no. of σ bonds = 8[4C-H;,3C-C;1C-O]
no. of π bonds = 3[2C-C; 1C-O]
ratio=8/3
6. Which one of the following is the likely bond angles of sulphur tetrafluoride molecule?
a) 120º,80 º
b) 109 º.28
c) 90 º
d) 89 º,117 º
Solution:
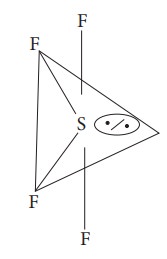
Normal bond angle in regular trigonal bipyramidal are 90º and 120 º
![]()
![]() due to l.p - b.p repulsion, bond angle reduced 89 º, 117 º
due to l.p - b.p repulsion, bond angle reduced 89 º, 117 º
7. Assertion: Oxygen molecule is paramagnetic.
Reason : It has two unpaired electron in its bonding molecular orbital
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) Both assertion and reason are false
Solution:
Correct Statement :
Oxygen molecule is paramagnetic Correct Reason
It has two unpaired electron in its antibond-ing molecular orbital.
Option (c) assertion true and reason false.
8. According to Valence bond theory, a bond between two atoms is formed when
a) fully filled atomic orbitals overlap
b) half filled atomic orbitals overlap
c) non- bonding atomic orbitals overlap
d) empty atomic orbitals overlap
Solution:
two half filled orbitals overlap.
9. In ClF3 ,NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are
a) sp3 hybridised
b) sp3 ,sp3 and sp2 respectively
c) sp2 hybridised
d) sp3d, sp3 and sp hybridised respectively
Solution:
ClF3 – Sp3d hybridisation
NF3 - Sp3 hybridisation
BF3 - Sp2 hybridisation
Option (d) is correct.
10. When one s and three p orbitals hybridise,
a) four equvivalent orbitals at 900 to each other will be formed
b) four equvivalent orbitals at 109º28' to each other will be formed.
c) four equivalent orbitals, that are lying the same plane will be formed
d) none of these
Solution:
Option (b) is correct Sp3 hybridisation.
Oribital geometry tetrahedron, bond angle 109º28'
11. Which of these represents the correct order of their increasing bond order.
a) C2 < C22- < O22- < O2
b) C2 2- < C2 + < O2 < O22-
c) O22- < O2 < C2 2- < C2 +
d) O22- < C2 +< O2 < C22-
Solution:
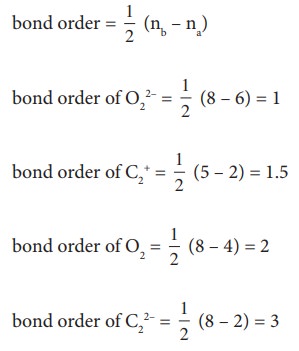
12. Hybridisation of central atom in PCl5 involves the mixing of orbitals.
a) s, px, py, dx2 , dx2-y2
b) s, px . py, pxy . dx2-y2
c) s, px , py , pz , dx2-y2
d) s, px , py , dxy , dx2-y2
Solution:
PCl5 – Sp3d hybridization
S, Px , Py , Pz and dx2 −y2
13. The correct order of O-O bond length in hydrogen peroxide, ozone and oxygen is
a) H2O2 > O3 >O2
b) O2 > O3 > H2 O2
c) O2 > H2 O2 > O 3
d) O3 > O2 > H2 O 2
Solution:
O2 > O3 > H2O2
2 > 1.5 > 1
14. Which one of the following is diamagnetic.?
a) O2
b) O22-
c) O+2
d) None of these
Solution:
O22– is diamagnetic. Additional two electrons paired in antibonding molecular orib-ital π*2Py and π*2 Pz
15. Bond order of a species is 2.5 and the number of electons in its bonding molecular orbital is formd to be 8 The no. of electons in its antibonding molecular orbital is
![]()
![]() a) three
a) three
b) four
c) Zero
d) can not be calculated form the given unformation.
Solution:
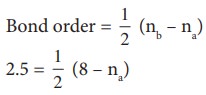
5 = 8 – na
na = 8-5 = 3
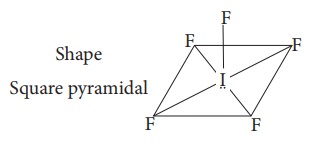
16. Shape and hybridisation of IF5 are
a) Trigonal bipyramidal, Sp3d2
b) Trigonal bipyramidal, Sp3d
c) Square pyramidal, Sp3d2
d) Octahedral, Sp3d2
Solution:
IF5 - 5 bond pair + 1 lone pair hybridisation Sp3d2
17. Pick out the incorrect statement from the following
a) Sp3 hybrid orbitals are equivalent and are at an angle of 109 º 28' with eachother
b) dsp2 hybrid orbitals are equivalent and bond angle between any two of them is 900
c) All five sp3d hybrid orbitals are not equivalent out of these five sp3d hybrid orbitals, three are at an angle of 120º, remainir two are perpendicular to the plane containing the other three
d) none of these
Solution:
Correct statement : All five Sp3d hybrid orbitals are equivalent
incorrect statement : Option (c)
18. The molecules having same hybridisation, shape and number of lone pairs of electons are
a) SeF4, XeO2 F2
b) SF4, Xe F2
c) XeOF4, TeF4
d) SeCl4, XeF4
Solution:
SeF4, XeO2F2 - Sp3d hybridisation,
option (a) T-shaped, one line pair on cen-tral atom.
19. In which of the following molecules / ions BF3, NO2-, H2 O the central atom is sp2 hybridised?
a) NH2- and H2O
b) NO2- and H2O
c) BF3 and NO2-
d) BF3 and NH2-
Solution:
H2O - Central atom Sp3 hybridised
NO2– - Central atom Sp2 hybridised
BF3 - Central atom Sp2 hybridised
NH2– - Central atom Sp3 hybridised option
(c) is correct.
20. Some of the following properties of two species, NO3- and H3O+ are described below. which one of them is correct?
a) dissimilar in hybridisation for the central atom with different structure.
b) isostructural with same hybridisation for the Central atom.
c) different hybridiration for the central atom with same structure
d) none of these
Solution:
NO3– - Sp2 hybridisation, planar
H3O+ - Sp3 hybridisation, pyramidal
option (a) is correct.
21. The types of hybridiration on the five carbon atom from right to left in the, 2,3 pentadiene.
a) sp3, sp2, sp, sp2, sp3
b) sp3, sp, sp, sp, sp3
c) sp2, sp, sp2,sp2, sp3
d) sp3, sp3, sp2, sp3, sp3
Solution:
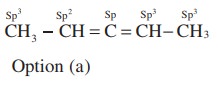
22. Xe F2 is isostructural with
a) SbCl2
b) BaCl2
c) TeF2
d) ICl2–
Solution:
XeF2 is isostructural with ICl2–
23. The percentage of s-character of the hybrid orbitals in methane, ethane, ethene and ethyne are respectively
a) 25, 25,33.3,50
b) 50,50,33.3,25
c) 50,25,33.3,50
d) 50,25,25,50
Solution:
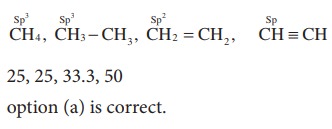
24. Of the following molecules, which have shape similar to carbondixide?
a) SnCl2
b) NO2
c) C2H2
d) All of these.
Solution:
Co2 - Linear
C2H2 - Linar
option (c) is correct.
25. According to VSEPR theory, the repulsion between different parts of electrons obey the order.
a) l.p – l.p > b.p–b.p> l.p–b.p
b) b.p–b.p> b.p–l.p> l.p–b.p
c) l.p–l.p> b.p–l.p > b.p–b.p
d) b.p–b.p> l.p–l.p> b.p–l.p
26. Shape of ClF3 is
a) Planar triangular
b) Pyramidal
c) 'T' Shaped
d) none of these
Solution:
ClF3 - Sp3d hybridisation, 'T' shaped
option (c) is correct.
27. Non- Zero dipole moment is shown by
a) CO2
b) p-dichlorobenzene
c) carbontetrachloride
d) water.
Solution:
28. Which of the following conditions is not correct for resonating structures?
a) the contributing structure must have the same number of unpaired electrons
b) the contributing structures should have similar energies
c) the resonance hybrid should have higher energy than any of the contributing structure.
d) none of these
Solution:
Correct statement is - the resonance hybrid should have lower energy than any of the contributing structure
Hence is correct statement is option (c)
29. Among the following, the compound that contains, ionic, covalent and Co-ordinate linkage is
a) NH4Cl
b) NH3
c) NaCl
d) none of these
30. CaO and NaCl have the same crystal structure and approximately the same radii. It U is the lattice energy of NaCl, the approximate lattice energy of CaO is
a) U
b) 2U
c) U/2
d) 4U
Related Topics