Chemical bonding - Valence Shell Electron Pair Repulsion (VSEPR) theory | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Valence Shell Electron Pair Repulsion (VSEPR) theory
Valence Shell Electron Pair Repulsion (VSEPR) theory
Lewis concept of structure of molecules deals with the relative position of atoms in the molecules and sharing of electron pairs between them. However, we cannot predict the shape of the molecule using Lewis concept. Lewis theory in combination with VSEPR theory will be useful in predicting the shape of molecules.
Important principles of VSEPR Theory are as follows:
1. The shape of the molecules depends on the number of valence shell electron pair around the central atom.
2. There are two types of electron pairs namely bond pairs and lone pairs. The bond pair of electrons are those shared between two atoms, while the lone pairs are the valence electron pairs that are not involved in bonding.
3. Each pair of valence electrons around the central atom repels each other and hence, they are located as far away as possible in three dimensional space to minimize the repulsion between them.
4. The repulsive interaction between the different types of electron pairs is in the following order.
lp - lp > lp - bp> bp-bp
lp- lone pair ; bp- bond pair
The lone pair of electrons are localised only on the central atom and interacts with only one nucleus whereas the bond pairs are shared between two atoms and they interact with two nuclei. Because of this the lone pairs occupy more space and have greater repulsive power than the bond pairs in a molecule.
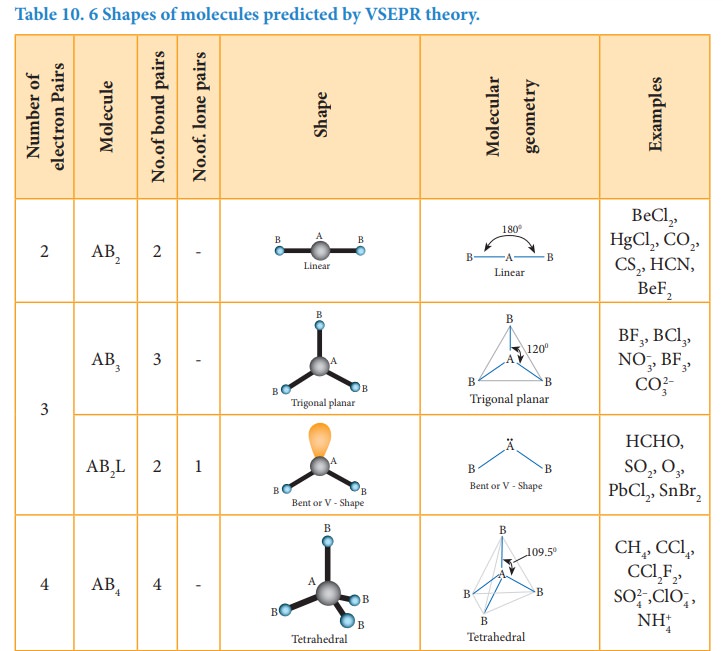
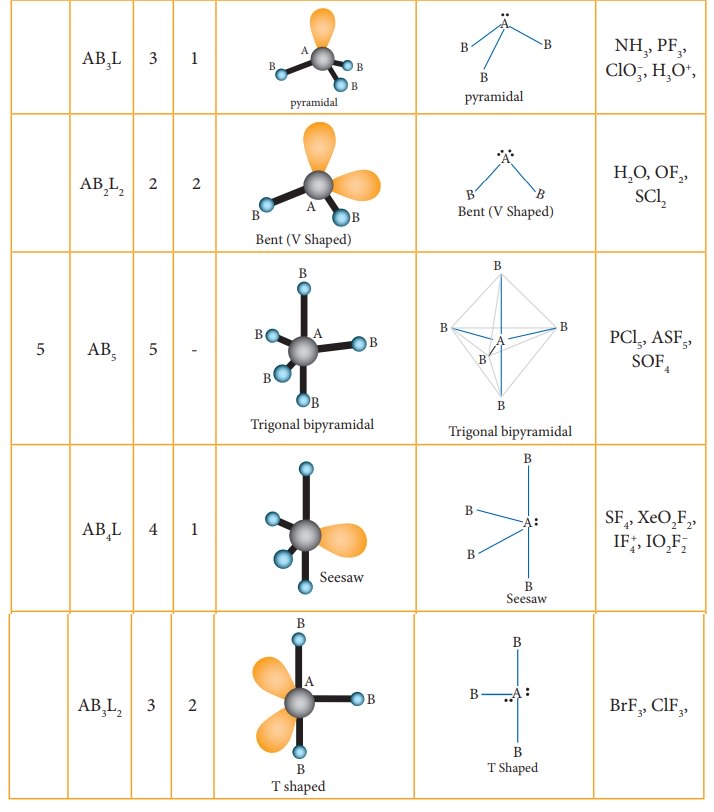
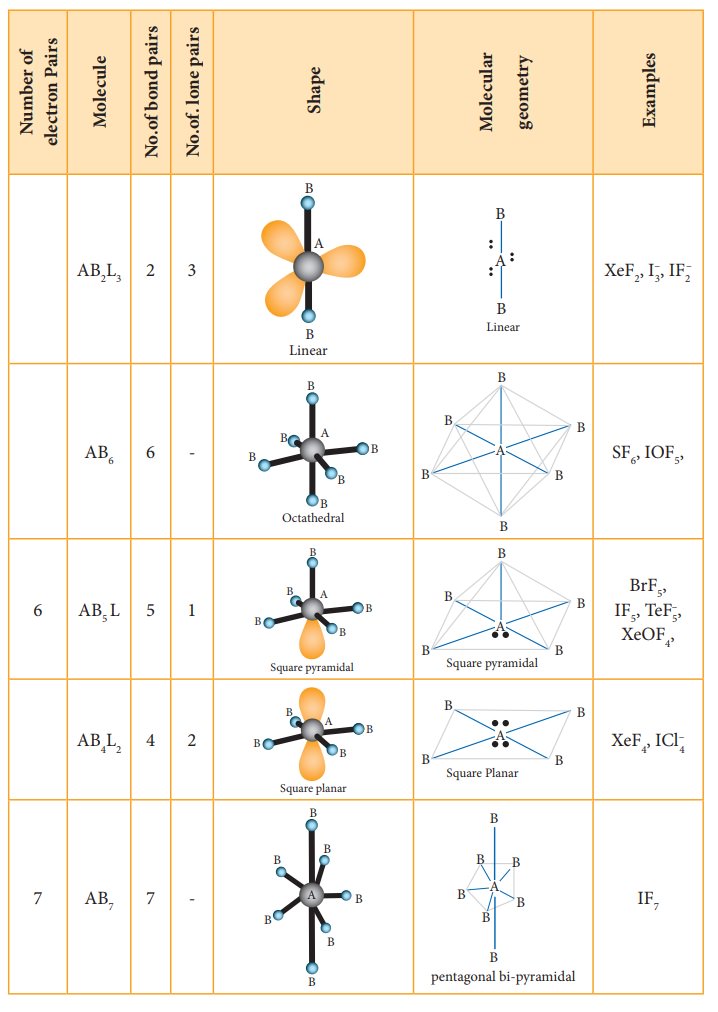
The following Table illustrates the shapes of molecules predicted by VSEPR theory. Consider a molecule ABx where A is the central atom and x represents the number of atoms of B covalently bonded to the central atom A. The lone pairs present in the atoms are denoted as L.
Related Topics