Book Back and Important Questions Answers | Chemistry - p-Block Elements-II: Answer the following questions | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
p-Block Elements-II: Answer the following questions
Chemistry : p-Block Elements-II
Answer the following questions:
1. What is inert pair effect?
In
p-block elements in the case of post -- transition elements belonging to group
number 13 to 16, the most stable oxidation state is two less than the group
oxidation state due to the inert tendency of ns electrons to take part in
chemical bonding. This effect is known as inert pair effect.
2. Chalcogens belongs to p-block. Give reason.
• Chalcogens have general
electronic configuration ns2, np4. The added electrons
filled in the p orbital. Hence it belong to p-block.
• The elements of chalgogens have
similar outer shell electronic configuration and differ only in the value of n
(principal quantum number).
3. Explain why fluorine always exhibit an oxidation state of -1?
Due
its more electronegativity it always accepts one electron in its valence shell.
Hence it always exhibits an oxidation state of −1. It will not lose electron
from its outermost shell. Therefore it will not exhibit positive oxidation
state.
4. Give the oxidation state of halogen in the following.
a) OF2 b) O2F2 c) Cl2O3 d) I2O4
a)
OF2 : −1
b)
O2F2 : −1
c)
Cl2O3 : +3
d)
I2O4 : +4
5. What are interhalogen compounds? Give examples.
Each
halogen combines with other halogens to form a series of compounds called inter
halogen compounds.
Example:
AB type - ClF
AB3 type - ClF3, ICl3
6. Why fluorine is more reactive than other halogens?
Due
to low bond dissociation energy and more electronegativity fluorine is more
reactive than other halogens.
7. Give the uses of helium.
1.
Helium and oxygen mixture is used by divers in place of air oxygen mixture.
This prevents the painful dangerous condition called bends.
2.
Helium is used to provide inert atmosphere in electric arc welding of metals
3.
Helium has lowest boiling point hence used in cryogenics.
4.
It is much less denser than air and hence used for filling air balloons
8. What is the hybridisation of iodine in IF7? Give its structure.
The
hybridisation of iodine in IF7 is sp3d3
Structure
of IF7 is pentagonal bipyramidal
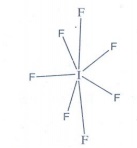
9. Give the balanced equation for the reaction between chlorine with cold NaOH and hot NaOH.
Cold NaOH
Chlorine
reacts with cold dilute alkali to give chloride and hypochlorite
Cl2
+ 2NaOH → NaOCl + NaCl + H2O
NaOCl
: Sodium hypo chlorite
Hot NaOH
Chlorine
reacts with hot concentrated alkali to give chlorides and chlorates
3Cl2 + 6NaOH → NaClO3 + 5NaCl + 3H2O
10. How will you prepare chlorine in the laboratory?
• Chlorine is prepared by the
action of conc. sulphuric acid on chlorides in presence of manganese dioxide.
4NaCl + MnO2 + 4H2SO4
→ Cl2 + MnCl2 + 4NaHSO4 + 2H2O
• Chlorine is liberated when
bleaching powder is treated with mineral acids
CaOCl2 + 2HCl → CaCl2 + H2O
+ Cl2
CaOCl2 + H2SO4
→ CaSO4 + H2O + Cl2
• Chlorine is prepared by oxidising
hydrochloric acid using various oxidising agents
PbO2
+ 4HCl → PbCl2 + 2H2O + Cl2
11. Give the uses of sulphuric acid.
i)
Sulphuric acid is used in the manufacture of fertilisers, ammonium sulphate and
super phosphates and other chemicals such as hydrochloric acid, nitric acid etc
ii)
It is used as a drying agent and also used in the preparation of pigments,
explosives etc
12. Give a reason to support that sulphuric acid is a dehydrating agent.
Sulphuric
acid removes water from many organic compounds.
C12H22O11
+ H2SO4 → 12C + H2SO4 . 11H2O
HCOOH + H2SO4 → CO + H2SO4 .H2O
13. Write the reason for the anamolous behaviour of Nitrogen.
• Small size
• High value of electronegativity
• High ionisation enthalpy
• Absence of d-orbital
14. Write the molecular formula and structural formula for the following molecules.
a) Nitric acid
b) dinitrogen pentoxide
c) phosphoric acid
d) phosphine
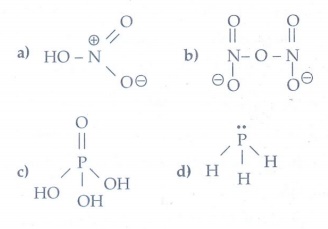
15. Give the uses of argon.
Argon prevents the oxidation of hot filament and
prolongs the life in filament bulbs
16. Write the valence shell electronic configuration of group-15 elements.
The
general valence shell electronic configuration of group−15 element is ns2np3
Nitrogen
2s22p3
Phosphorous
3s23p3
Arsenic
4s24p3
Antimony
5s25p3
Bismuth
6s26p3
17. Give two equations to illustrate the chemical behaviour of phosphine.
• When phosphine is heated with air
or oxygen it burns to give meta phosphoric acid.
4PH3
+ 8O2 __Δ_→ P4O10 + 6H2O
P4O10
+ 6H2O __Δ_ → 4HPO3 + 4H2O
• Phosphine precipitates metal from
their salt solutions.
3AgNO3
+ PH3 → Ag3P + 3HNO3
18. Give a reaction between nitric acid and a basic oxide.
Nitric
acid reacts with basic oxides to form salts and water
3FeO
+ 10HNO3 → 3Fe(NO3)3 + NO + 5 H2O
19. What happens when PCl5 is heated?
On
heating phosphorous pentachloride decomposes into phosphorus trichloride and
chlorine.
PCl5
→ PCl3 + Cl2
20. Suggest a reason why HF is a weak acid, whereas binary acids of the all other halogens are strong acids.
Hydrochloric,
hydrobromic and hydroiodic acids are almost completely ionized and they are
strong acids. 0.l mM solution of HF ionized only 10%, therefore it is weak
acidic.
21. Deduce the oxidation number of oxygen in hypofluorous acid – HOF.
HOF
= +l + x – l = 0
Oxygen exhibits zero state in hypofluorous acid
22. What type of hybridisation occur in
a) BrF5 b) BrF3
Answer:
a)
BrF5 - sp3d2
b)
BrF3 - sp3d
23. Complete the following reactions.
1. NaCl + MnO2 + H2SO4 →
2. NaNO2 + HCl →
2. IO3− + I− + H+ →
3. I2 + S2O32− →
4. P4 + NaOH + H2O →
5. AgNO3 + PH3 →
6. Mg + HNO3 →
7. KClO3 →Δ hot conc.
8. Cu + H2 SO4→
9. Sb + Cl2 →
10. HBr + H2SO4 →
11. XeF6 + H2O→
12. XeO64− + Mn2+ + H+ →
13. XeOF4 + SiO2 →
14. Xe + F2 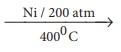
Answer:
1.
4NaCl + MnO2 + 4H2SO4 → Cl2 + MnCl2
+ 4NaHSO4 + 2H2O
2.
NaNO2 + HCl → NaCl + HNO2
3.
IO3− +5I− + 6H+ → 3I2 +
3H2O
4.
I2 + 2S2O32− → S4O62−
+ 2I−
5.
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3↑
6.
3AgNO3 + PH3 → Ag3P + 3HNO3
7.
4Mg + 10HNO3 → 4Mg(NO3)2 + N2O + 5H2O
8.
2KClO3 ___Δ Hot conc.__→
2KCl + 3O2
9.
Cu + 2H2SO4 → CuSO4 + 2H2O + SO2↑
10.
2Sb + 3Cl2 → 2SbCl3
11.
2HBr + H2SO4 → SO2 + 2H2O + Br2
12.
XeF6 + 3H2O → XeO3 + 6HF
13.
5XeO64− + 2Mn2+ + 14H+ → 2MnO4−
+ 5XeO3+ 7H2O
14.
2XeOF4 + SiO2 → 2XeO2F2 + SiF4
15.
Xe + 3F2 __Ni/200 atm_400oC__→
XeF6
EVALUATE YOURSELF:
l. Write the products formed in
the reaction of nitric acid (both dilute and concentrated) with zinc.
Zinc
reacts with HNO3 at various concentrations and it gives different
products.
4
Zn + 10 HNO3 (dil)
↓
4
Zn (NO3)2 [Zinc
nitrate] + N2O [Nitrous
oxide] +
5 H2O
4
Zn + 10 HNO3 (very dilute)
↓
4 Zn (NO3)2 [Zinc nitrate] + NH4NO3 [Ammonium
nitrate] + 3H2O
Related Topics