Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Properties and Uses of phosphorus
Properties of phosphorus
Phosphorus is highly
reactive and has the following important chemical properties
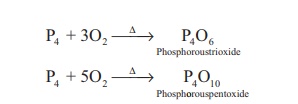
Reaction with oxygen: Yellow phosphorus
readily catches fire in air giving dense white fumes of phosphorus
pentoxide. Red phosphorus also reacts with oxygen on heating to give phosphorus
trioxide or phosphorus pentoxide.
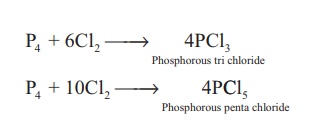
Reaction with chlorine: Phosphorus reacts with chlorine to form tri and penta chloride. Yellow phosphorus reacts violently at room temperature, while red phosphorous reacts on heating
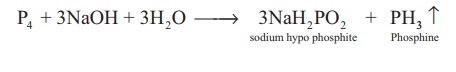
Reaction with alkali: Yellow phosphorous
reacts with alkali on boiling in an inert atmosphere liberating
phosphine. Here phosphorus act as reducing agent.
Reaction with nitric
acid: When
phosphorous is treated with conc. nitric acid it is oxidised to phosphoric
acid. This reaction is catalysed by iodine crystals.

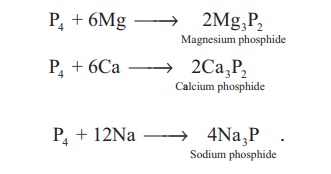
Reaction with metals: Phosphorous reacts with
metals like Ca and Mg to give phosphides.. Metals like sodium and potassium
react with phosphorus vigorously.
Uses of phosphorous:
·
The red phosphorus is used in the match boxes
·
It is also used for the production of certain alloys such as
phosphor bronze
Related Topics