Preparation, Properties, Structure, Uses - Phosphorous trichloride | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Phosphorous trichloride
Phosphorous trichloride:
Preparation:
When a slow stream of chlorine is passed over white phosphorous, phosphorous trichloride is formed. It can also be obtained by treating white phosphorous with thionyl chloride.
P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2
Properties
When phosphorous trichloride is hydrolysed with cold water it gives phosphorous acid.
PCl3 + 3H2O → H3PO3 + 3HCl
This reaction involves the coordination of a water molecule using a vacant 3d orbital on the phosphorous atom following by elimination of HCl which is similar to hydrolysis of SiCl4.
PCl3 + H2O → PCl3.H2O → P(OH)Cl2 + HCl
This reaction is followed by two more steps to give P(OH)3 or H3PO3.
HPOC12 + H2O → H2PO2Cl + HCl
H2PO2Cl + H2O → H2PHO3 + HCl
Similar reactions occurs with other molecules that contains alcohols and carboxylic acids.
C2H5OH + PCl3 → C2H5Cl + H3PO3
C2H5COOH + PCl3 → C2H5COCl + H3PO3
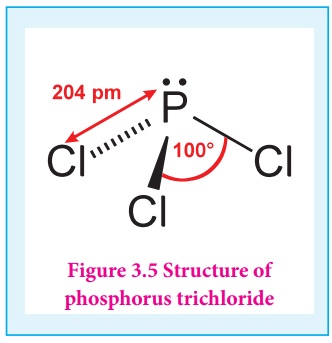
Uses of phosphorus trichloride:
Phosphorus trichloride is used as a chlorinating agent and for the preparation of H3PO3.
Related Topics