Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Allotropic forms of phosphorus
Allotropic
forms of phosphorus:
Phosphorus has several
allotropic modification of which the three forms namely white, red and black
phosphorus are most common.
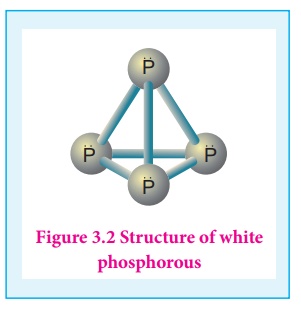
The freshly prepared
white phosphorus is colourless but becomes pale yellow due to formation of a
layer of red phosphorus upon standing. Hence it is also known as yellow
phosphorus. It is poisonous in nature and has a characteristic garlic smell. It
glows in the dark due to oxidation which is called phosphorescence. Its ignition
temperature is very low and hence it undergoes spontaneous combustion in air at
room temperature to give P2O5.
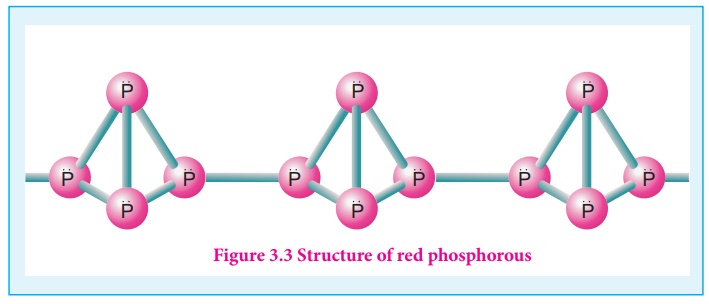
The white phosphorus can
be changed into red phosphorus by heating it to 420 ⁰C in the absence of air
and light. Unlike white phosphorus it is not poisonous and does not show
Phosphorescence. It also does not ignite at low temperatures. The red
phosphorus can be converted back into white phosphorus by boiling it in an
inert atmosphere and condensing the vapour under water.
The phosphorus has a
layer structure and also acts as a semiconductor. The four atoms in phosphorus
have polymeric structure with chains of P4 linked tetrahedrally.
Unlike nitrogen P≡P
is less stable than P-P single bonds. Hence, phosphorus atoms are linked
through single bonds rather than triple bonds. In addition to the above two
more allotropes namely scarlet and violet phosphorus are also known for
phosphorus.
Related Topics