Preparation, Properties, Structure, Uses - Sulphur dioxide | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Sulphur dioxide
Sulphur
dioxide
Preparation
From sulphur: A large-scale production
of sulphur dioxide is done by burning sulphur in air.
About 6-8% of sulphur is
oxidised to SO3.
S + O2 → SO2
2S + 3O2 → 2SO3
From sulphides: When sulphide ores such
as galena (PbS), zinc blende (ZnS) are roasted in air, sulphur dioxide
is liberated. Large amounts of sulphur dioxide required for manufacturing of
sulphuric acid and other industrial purpose is prepared by this method.
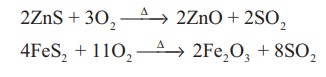
Laboratory preparation: Sulphur dioxide is
prepared in the laboratory treating a metal or metal sulphite with
sulphuric acid
Cu + 2H2SO4
→ CuSO4 + SO2 + 2H2O
SO3-
+ 2H+ → H2O + SO2
Properties:
Sulphur dioxide gas is
found in volcanic eruptions. A large amount of sulphur dioxide gas is released
into atmosphere from power plants using coal and oil and copper melting plants.
It is a colourless gas with a suffocating odour. It is highly soluble in water
and it is 2.2 times heavier than air. Sulphur dioxide can be liquefied (boiling
point 263 K) at 2.5 atmospheric pressure and 288 K.
Chemical properties
Sulphur dioxide is an
acidic oxide. It dissolves in water to give sulphurous acid.
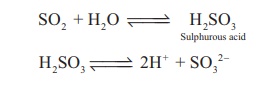
SO2 + H2O
↔ H2SO3 (Sulphurous acid)
H2SO3
↔ 2H+ + SO32−
Reaction with sodium
hydroxide and sodium carbonate: Sulphur dioxide reacts with sodium hydroxide
and sodium carbonate to form sodium bisulphite and sodium sulphite
respectively.
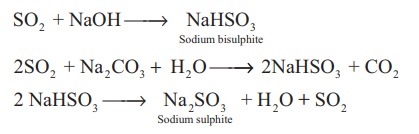
SO2 + NaOH → NaHSO3 (Sodium bisulphite)
2SO2 + Na2CO3
+ H2O → 2NaHSO3 + CO2
2 NaHSO3 → Na2SO3(Sodium
sulphite) + H2O + SO2
Oxidising property: Sulphur dioxide,
oxidises hydrogen sulphide to sulphur and magnesium to magnesium oxide.
2H2S + SO2
→ 3S + 2H2O
2Mg + SO2 → 2MgO
+ S
Reducing property: As it can readily be
oxidised, it acts as a reducing agent. It reduces chlorine into
hydrochloric acid.
SO2 + 2H2O
+ Cl2 → H2SO4 + 2HCl
It also reduces
potassium permanganate and dichromate to Mn2+ and Cr3+ respectively.
2KMnO4 + 5SO2
+ 2H2O → K2SO4 + 2MnSO4 + 2H2SO4
K2Cr2O7
+ 3SO2 + H2SO4 → K2SO4 + Cr2 (SO4
)3 + H2O
Reaction with oxygen: Sulphur dioxide is oxidised to sulphur trioxide upon heating with oxygen at high temperature. This reaction is used for the manufacture of sulphuric acid by contact process.
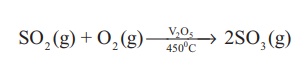
Bleaching action of
sulphur dioxide: In presence of water, sulphur dioxide bleaches coloured wool,
silk, sponges and straw into colourless due to its reducing property.
SO2 + 2H2O
→ 2 H2SO4 + 2(H)
(Coloured) X + 2(H) → XH2(Colourless)
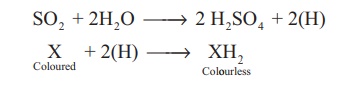
However, the bleached
product (colourless) is allowed to stand in air, it is reoxidised by
atmospheric oxygen to its original colour. Hence bleaching action of sulphur
dioxide is temporary.
Uses:
·
Sulphur dioxide is used in bleaching hair, silk, wool etc...
·
It can be used for disinfecting crops and plants in agriculture.
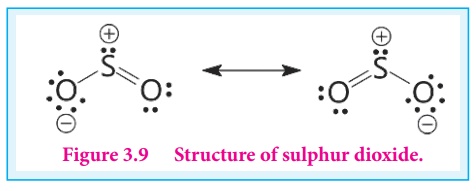
Structure of sulphur dioxide:
In sulphur dioxide, sulphur
atom undergoes sp2 hybridisation. A double bond arises between S and
O is due to pπ- dπ overlapping.
Related Topics