Preparation, Physical and Chemical Properties, Manufacture, Structure, Uses - Chlorine | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Chlorine
Chlorine is highly
reactive hence it doesn’t occur free in nature. It is usually distributed as
various metal chlorides. The most important chloride is sodium chloride which
occurs in sea water.
Preparation:
Chlorine is prepared by
the action of conc. sulphuric acid on chlorides in presence of manganese
dioxide.
4NaCl + MnO2
+ 4H2SO4 → Cl2 + MnCl2 + 4NaHSO4
+ 2H2O
It can also be prepared
by oxidising hydrochloric acid using various oxidising agents such as manganese
dioxide, lead dioxide, potassium permanganate or dichromate.
PbO2 + 4HCl →
PbCl2 + 2H2O + Cl2
MnO2 + 4HCl →
MnCl2 + 2H2O + Cl2
2KMnO4 +
16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
K2Cr2O7
+ 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
When bleaching powder is
treated with mineral acids chlorine is liberated
CaOCl2 + 2HCl
→ CaCl2 + H2O + Cl2
CaOCl2 + H2SO4
→ CaSO4 + H2O + Cl2
Manufacture of chlorine:
Chlorine is manufactured
by the electrolysis of brine in electrolytic process or by oxidation of HCl by
air in Deacon’s process.
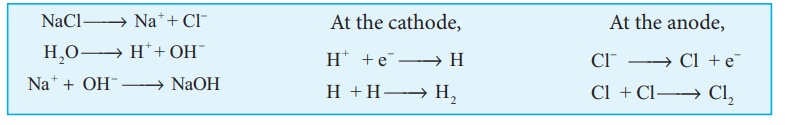
Electrolytic process: When a solution of brine
(NaCl) is electrolysed, Na+ and Cl ions are formed.
Na+ ion reacts with OH- ions of water and forms sodium hydroxide.
Hydrogen and chlorine are liberated as gases.
Deacon’s process: In this process a
mixture of air and hydrochloric acid is passed up a chamber containing a
number of shelves, pumice stones soaked in cuprous chloride are placed. Hot
gases at about 723 K are passed through a jacket that surrounds the chamber.
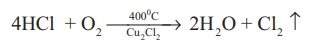
The chlorine obtained by
this method is dilute and is employed for the manufacture of bleaching powder.
The catalysed reaction is given below,
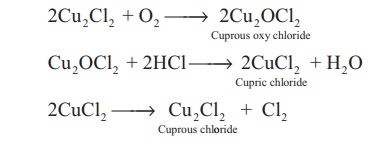
2Cu2Cl2
+ O2 → 2Cu2OCl2 Cuprous oxy chloride
Cu2OCl2
+ 2HCl → 2CuCl2 Cupric chloride + H2O
2CuCl2 → Cu2Cl2
Cuprous chl + Cl2
Physical properties:
Chlorine is a greenish
yellow gas with a pungent irritating odour. It produces headache when inhaled
even in small quantities whereas inhalation of large quantities could be fatal.
It is 2.5 times heavier than air.
Chlorine is soluble in
water and its solution is referred as chlorine water. It deposits greenish
yellow crystals of chlorine hydrate (Cl2.8H2O). It can be
converted into liquid (Boiling point – 34.6° C) and yellow crystalline solid
(Melting point -102° C)
Chemical properties:
Action with metals and
non-metals: It reacts with metals and non metals to give the corresponding
chlorides.
2Na + Cl2 → 2NaCl
2Fe + 3Cl2 → 2FeCl3
2Al + 3Cl2 → 2AlCl3
Cu + Cl2 → CuCl2
H2 + Cl2
→ 2HCl ; H = − 44kCal
2B + 3Cl2 → 2BCl3
2S + Cl2 → S2Cl2
disulphur dichloride
P4 + 6Cl2
→ 4PCl3
2As + 3Cl2 → 2AsCl3
2Sb + 3Cl2 → 2SbCl3
Affinity for hydrogen : When burnt with
turpentine it forms carbon and hydrochloric acid.
C10H16
+ 8Cl2 → 10C + 16HCl
It forms dioxygen when
reacting with water in presence of sunlight. When chlorine in water is exposed
to sunlight it loses its colour and smell as the chlorine is converted into
hydrochloric acid.
2Cl2 + 2H2O
→ O2 + 4HCl
Chlorine reacts with
ammonia to give ammonium chloride and other products as shown below:
With excess ammonia,
2NH3 + 3Cl2
→ N2 + 6HCl
6HCl + 6 NH3 → 6 NH4Cl
overall reaction
8NH3 + 3Cl2
→ N2 + 6 NH4Cl
With excess chlorine,
NH3 + 3Cl2
→ NCl3 + 3HCl
3HCl + 3NH3 → 3NH4Cl
overall reaction
4NH3 + 3Cl2
→ NCl3 + 3NH4Cl
Chlorine oxidises
hydrogen sulphide to sulphur and liberates bromine and iodine from iodides and
bromides. However, it doesn't oxidise fluorides
H2S + Cl2
→ 2HCl + S
Cl2 + 2KBr → 2KCl + Br2
Cl2 + 2KI → 2KCl + I2
Reaction with alkali: Chlorine reacts with
cold dilute alkali to give chloride and hypochlorite while with hot
concentrated alkali chlorides and chlorates are formed.
Cl2 + H2O
→ HCl + HOCl
HCl + NaOH → NaCl + H2O
HOCl + NaOH → NaOCl + H2O
overall reaction
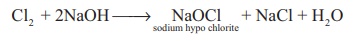
Cl2 + 2NaOH → NaOCl
sodium hypo chlorite + NaCl + H2O
( Cl2 + H2O → HCl + HOCl)
× 3
( HCl + NaOH → NaCl + H2O) × 3
( HOCl + NaOH → NaOCl + H2O) × 3
3NaOCl → NaClO3 + 2NaCl
overall reaction
3Cl2 + 6NaOH → NaClO3 + 5NaCl + 3H2O
Oxidising and bleaching
action: Chlorine
is a strong oxidising and bleaching agent because of the nascent oxygen.
H2O + Cl2
→ HCl + HOCl Hypo chlorous acid
HOCl → HCl + (O)
Colouring matter +
Nascent oxygen → Colourless oxidation product
The bleaching of
chlorine is permanent. It oxidises ferrous salts to ferric, sulphites to
sulphates and hydrogen sulphide to sulphur.
2FeCl2 + Cl2
→ 2FeCl3
Cl2 + H2O
→HCl + HOCl
2FeSO4 + H2SO4
+ HOCl →Fe2 (SO4 )3 + HCl + H2O
overall reaction
2FeSO4 + H2SO4
+ Cl2 →Fe2 (SO4 )3 + 2HCl
Cl2 + H2O
→HCl + HOCl
Na2SO3
+ HOCl →Na2SO4 + HCl
overall reaction
Na2SO3
+ H2O + Cl2 →Na2SO4 + 2HCl
Cl2 + H2S
→2HCl + S
Preparation of bleaching
powder: Bleaching
powder is produced by passing chlorine gas through dry slaked lime
(calcium hydroxide).
Ca(OH)2 + Cl2
→ CaOCl2 + H2O
Displacement redox
reactions: Chlorine displaces bromine from bromides and iodine from iodide
salts.
Cl2 + 2KBr → 2KCl
+ Br2
Cl2 + 2KI →2KCl
+ I2
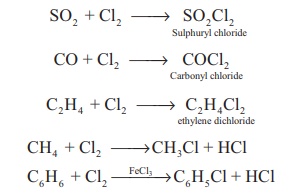
Formation of addition
compounds: Chlorine forms addition products with sulphur dioxide,
carbon monoixde and ethylene. It forms substituted products with
alkanes/arenes.
SO2 + Cl2
→ SO2Cl2Sulphuryl chloride
CO + Cl2 → COCl2Carbonyl
chloride
C 2 H4
+ Cl2 → C2H4Cl2ethylene
dichloride
CH4 + Cl2
→CH3Cl + HCl
C6H6
+ Cl2 →FeCl3→C6H5Cl + HCl
Uses of chlorine:
·
It is used in
·
Purification of drinking water
·
Bleaching of cotton textiles, paper and rayon
·
Extraction of gold and platinum
Related Topics