Preparation, Properties - Trends in physical and chemical properties of hydrogen halides | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Trends in physical and chemical properties of hydrogen halides
Trends
in physical and chemical properties of hydrogen halides:
Preparation:
Direct combination is a
useful means of preparing hydrogen chloride. The reaction between hydrogen and
fluorine is violent while the reaction between hydrogen and bromine or hydrogen
and iodine are reversible and don’t produce pure forms.
Displacement reactions:
Concentrated sulphuric
acid displaces hydrogen chloride from ionic chlorides. At higher temperatures
the hydrogen sulphate formed react with further ionic chloride. Displacement
can be used for the preparation of hydrogen fluorides from ionic fluorides.
Hydrogen bromide and hydrogen iodide are oxidised by concentrated sulphuric
acid and can’t be prepared in this method.
Hydrolysis of phosphorus trihalides:
Gaseous hydrogen halides
are produced when water is added in drops to phosphorus tri halides except
phosphorus trifluoride.
PX3 + 3H2O
→ H3PO3 + 3HX
Hydrogen bromide may be
obtained by adding bromine dropwise to a paste of red phosphorous and water
while hydrogen iodide is conveniently produced by adding water dropwise to a
mixture of red phosphorous and iodine.
2P + 3X2 → 2PX3
2PX3 + 3H2O
→ H3PO3 + 3HX
(where X=Br or I)
Any halogen vapours
which escapes with the hydrogen halide is removed by passing the gases through
a column of moist red phosphorous.
From covalent hydrides:
Halogens are reduced to
hydrogen halides by hydrogen sulphide.
H2S + X2
→ 2HX + S
Hydrogen chloride is
obtained as a by-product of the reactions between hydrocarbon of halogens.
Table 3.4: General Properties:
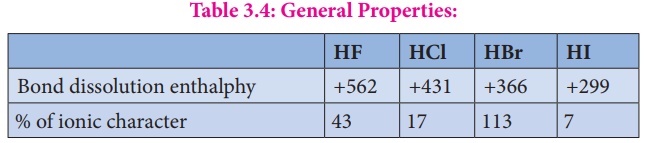
In line with the
decreasing bond dissociation enthalpy, the thermal stability of hydrogen
halides decreases from fluoride to iodide.
For example, Hydrogen
iodide decomposes at 400° C while hydrogen fluoride and hydrogen chloride are
stable at this temperature.
At room temperature,
hydrogen halides are gases but hydrogen fluoride can be readily liquefied. The
gases are colourless but, with moist air gives white fumes due to the
production of droplets of hydrohalic acid. In HF, due to the presence of strong
hydrogen bond it has high melting and boiling points. This effect is absent in
other hydrogen halides.
Acidic properties:
The hydrogen halides are extremely soluble in water due to the ionisation.
HX + H2O → H3O+ + X−
(X – F, Cl, Br, or I)
Solutions of hydrogen
halides are therefore acidic and known as hydrohalic acids. Hydrochloric,
hydrobromic and hydroiodic acids are almost completely ionised and are
therefore strong acids but HF is a weak acid i.e. 0.1mM solution is only 10%
ionised, but in 5M and 15M solution HF is stronger acid due to the equilibrium.
HF + H2O ↔ H3O+
+ F-
HF + F ↔ HF2-
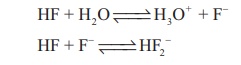
At high concentration,
the equilibrium involves the removal of fluoride ions is important. Since it
affects the dissociation of hydrogen fluoride and increases and hydrogen ion
concentration Several stable salts NaHF2, KHF2 and NH4HF2
are known. The other hydrogen halides do not form hydrogen dihalides.
Hydrohalic acid shows
typical acidic properties. They form salts with acids, bases and reacts with
metals to give hydrogen. Moist hydrofluoric acid (not dry) rapidly react with
silica and glass.
SiO2 + 4HF → SiF4
+ 2H2O
Na2SiO3
+ 6HF → Na2SiF6 + 3H2O
Oxidation: Hydrogen iodide is
readily oxidised to iodine hence it is a reducing agent.
2HI ↔ H+
+ I2 + 2e-
Acidic solution of
iodides is readily oxidised. A positive result is shown by liberation of iodine
which gives a blue-black colouration with starch.
Hydrogen bromide is more
difficult to oxidise than HI. HBr reduces slowly H2SO4
into SO2
2HBr + H2 SO4
→ 2H2O + Br2 + SO2
But hydrogen iodide and
ionic iodides are rapidly reduced by H2SO4 into H2S
and not into SO2.
8HI + H2SO4
→ 4H2O + 4I2 + H2S
Reducing property of
hydrogen iodide can be also explained by using its reaction with alcohols into
ethane. It converts nitric acid into nitrous acid and dinitrogen dioxide into
ammonium.
Hydrogen chloride is
unaffected by concentrated sulphuric aid by only strong oxidising agents like
MnO2, potassium permanganate or potassium chloride.
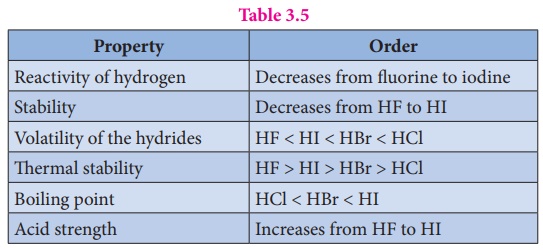
Table 3.5
Related Topics