Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Structure of oxides and oxoacids of phosphorus
Structure
of oxides and oxoacids of phosphorus
Phosphorous forms
phosphorous trioxide, phosphorous tetra oxide and phosphorous pentaoxides
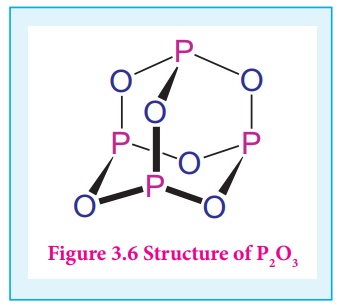
In phosphorous trioxide
four phosphorous atoms lie at the corners of a tetrahedron and six oxygen atoms
along the edges. The P-O bond distance is 165.6 pm which is shorter than the
single bond distance of P-O (184 pm) due to pπ-dπ bonding and results in
considerable double bond character.
In P4O10
each P atoms form three bonds to oxygen atom and also an additional coordinate
bond with an oxygen atom.
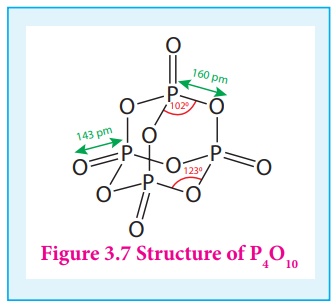
Terminal coordinate P-O
bond length is 143 pm, which is less than the expected single bond distance.
This may be due to lateral overlap of filled p orbitals of an oxygen atom with
empty d orbital on phosphorous.
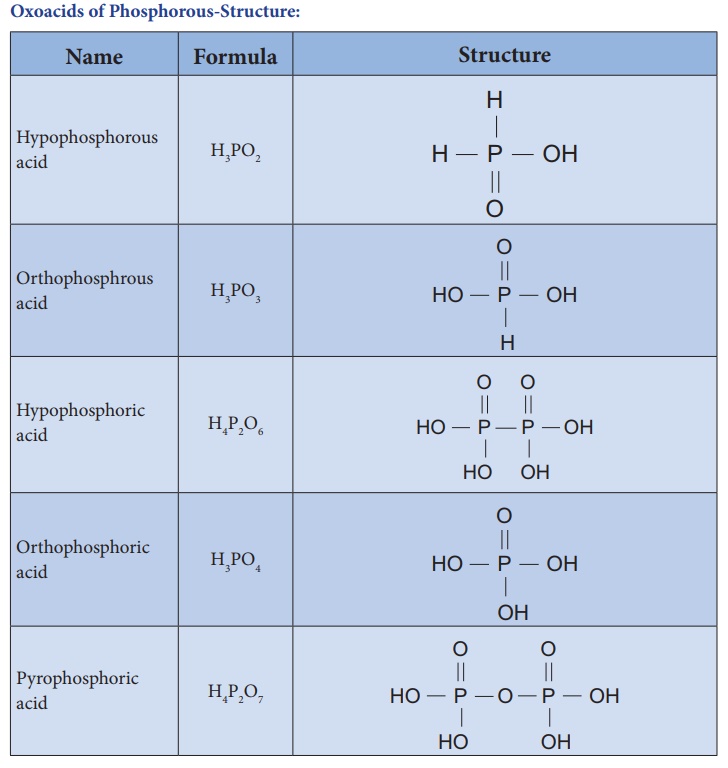
Oxoacids of Phosphorous-Structure:
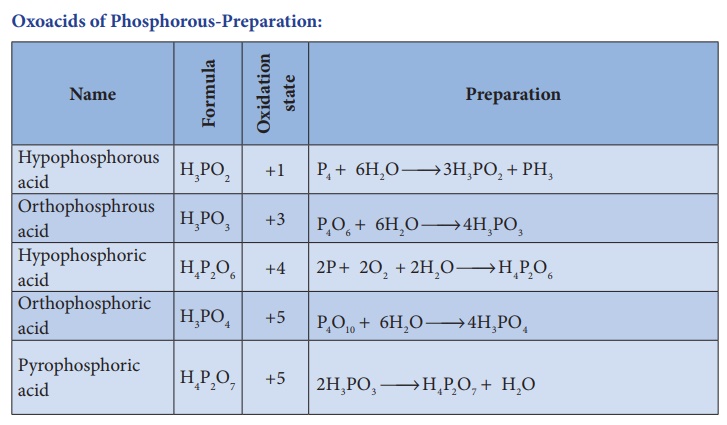
Oxoacids of Phosphorous-Preparation:
Related Topics