Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Oxides of halogen
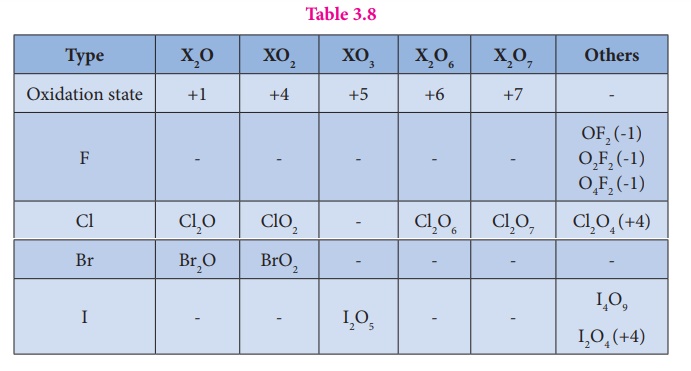
Fluorine reacts readily with oxygen and forms difluorine oxide and difluorine dioxide where it has a -1 oxidation state.
Oxides
of halogen
Fluorine reacts readily
with oxygen and forms difluorine oxide (F2O) and difluorine dioxide
(F2O2) where it has a -1 oxidation state. Other halogens
do not react with oxygen readily. But the following oxides can be prepared by
some indirect methods. Except fluorine all the other halogens have positive
oxidation states.
Table 3.8
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
12th Chemistry : UNIT 3 : p-Block Elements-II : Oxides of halogen |
Related Topics
12th Chemistry : UNIT 3 : p-Block Elements-II