Preparation, Properties, Structure, Uses - Phosphine | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Phosphine
Phosphine
(PH3)
Phosphine is the most
important hydride of phosphorous
Preparation:
Phosphine is prepared by
action of sodium hydroxide with white phosphorous in an inert atmosphere of
carbon dioxide or hydrogen.
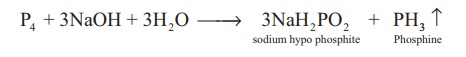
Phosphine is freed from phosphine dihydride(P2H4) by passing through a freezing mixture. The dihydride condenses while phosphine does not.
Phosphine can also
prepared by the hydrolysis of metallic phosphides with water or dilute mineral
acids.
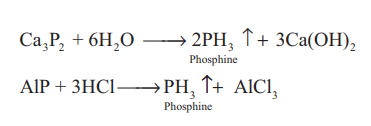
Phosphine is prepared in
pure form by heating phosphorous acid.

A pure sample of
phosphine is prepared by heating phosphonium iodide with caustic soda solution.
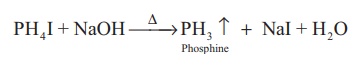
Physical properties:
It is colourless,
poisonous gas with rotten fish smell. It is slightly soluble in water and is
neutral to litmus test. It condenses to a colourless liquid at 188 K and
freezes to a solid at 139.5 K .
Chemical properties:
Thermal stability: Phosphine decomposes
into its elements when heated in absence of air at 317 K or when
electric current is passed through it.
4PH3 → 317K →P4 + 6H2
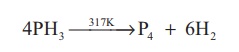
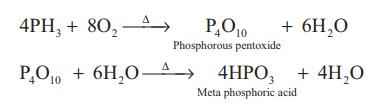
Combustion: When phosphine is heated
with air or oxygen it burns to give meta phosphoric acid.
Basic nature: Phosphine is weakly
basic and forms phosphonium salts with halogen acids.
PH3 + HI → PH4I
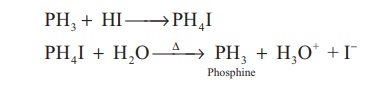
It reacts with halogens
to give phosphorus penta halides.
PH3 + 4Cl2 → PCl5 + 3HCl
Reducing property : Phosphine precipitates
some metal from their salt solutions.
3AgNO3 + PH3
→ Ag3P + 3HNO3
It forms coordination
compounds with lewis acids such as boron trichloride.
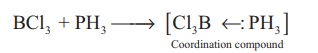
Structure:
In phosphine, phosphorus
shows sp3 hybridisation.
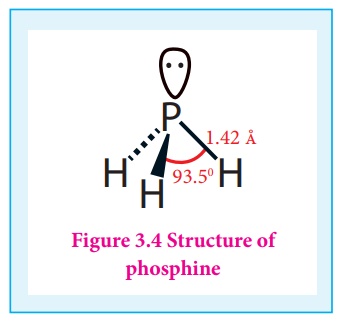
Three orbitals are
occupied by bond pair and fourth corner is occupied by lone pair of electrons.
Hence, bond angle is reduced to 94°. Phosphine has a pyramidal shape.
Uses of phosphine:
Phosphine is used for
producing smoke screen as it gives large smoke. In a ship, a pierced container
with a mixture of calcium carbide and calcium phosphide, liberates phosphine
and acetylene when thrown into sea. The liberated phosphine catches fire and
ignites acetylene. These burning gases serves as a signal to the approaching ships.
This is known as Holmes signal.
Related Topics