Introduction | Chemistry - Transition and Inner Transition Elements | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Transition and Inner Transition Elements
TRANSITION
AND INNER TRANSITION ELEMENTS
INTRODUCTION
Generally the metallic
elements that have incompletely filled d or f sub shell in the neutral or
cationic state are called transition metals. This definition includes
lanthanoides and actinides. However, IUPAC defines transition metal as an
element whose atom has an incomplete d sub shell or which can give rise to
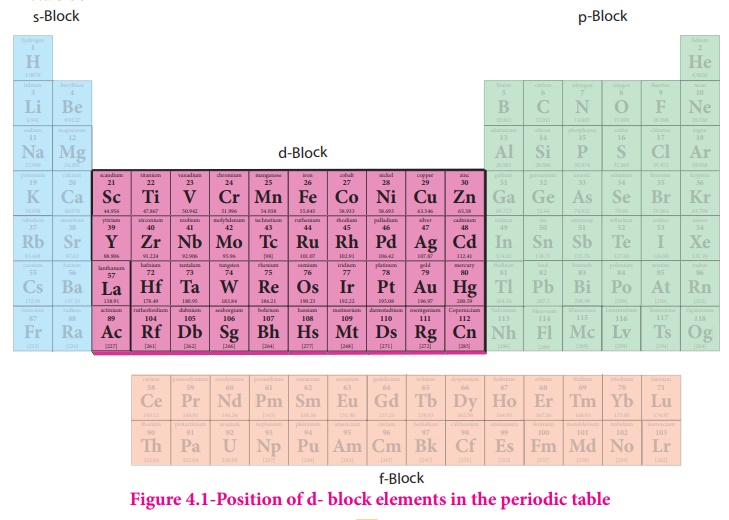
cations with an incomplete d sub shell. They occupy the central position of the
periodic table, between s and p block elements, and their properties are
transitional between highly reactive metals of s block and elements of p block
which are mostly non metals. Except group- 11 elements all transition metals
are hard and have very high melting point.
Transition metals, iron
and copper play an important role in the development of human civilization.
Many other transition elements also have important applications such as
tungsten in light bulb filaments, titanium in manufacturing artificial joints,
molybdenum in boiler plants, platinum in catalysis etc. They also play vital
role in living system, for example iron in hemoglobin, cobalt in vitamin B12
etc.,
In this unit we study
the general trend in properties of d block elements with specific reference to
3d series, their characteristics, chemical reactivity, some important compounds
KMnO4 and K2Cr2O7, we also discuss
the f-block elements later in this unit.
Related Topics