Chemistry - General trend in properties of Transition Elements | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
General trend in properties of Transition Elements
General
trend in properties:
1. Metallic behavior:
All the transition
elements are metals. Similar to all metals the transition metals are good
conductors of heat and electricity. Unlike the metals of Group-1 and group-2,
all the transition metals except group 11 elements are hard. Of all the known
elements, silver has the highest electrical conductivity at room temperature.
Most of the transition
elements are hexagonal close packed, cubic close packed or body centrered cubic
which are the characteristics of true metals.
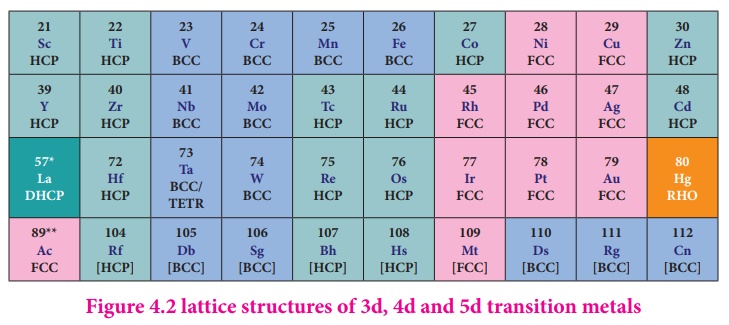
As we move from left to
right along the transition metal series, melting point first increases as the
number of unpaired d electrons available for metallic bonding increases, reach
a maximum value and then decreases, as the d electrons pair up and become less
available for bonding.
For example, in the
first series the melting point increases from Scandium (m.pt 1814K) to a
maximum of 2183 K for vanadium, which is close to 2180K for chromium. However,
manganese in 3d series and Tc in 4d series have low melting point. The maximum
melting point at about the middle of transition metal series indicates that d5
configuration is favorable for strong interatomic attraction. The following
figure shows the trends in melting points of transition elements.
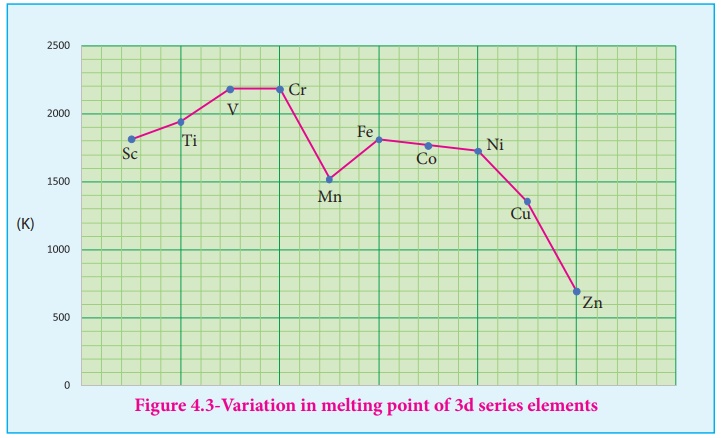
2. Variation of atomic and ionic size:
It is generally expected
a steady decrease in atomic radius along a period as the nuclear charge
increases and the extra electrons are added to the same sub shell. But for the
3d transition elements, the expected decrease in atomic radius is observed from
Sc to V , thereafter up to Cu the atomic radius nearly remains the same. As we
move from Sc toZn in 3d series the extra electrons are added to the 3d
orbitals, the added 3d electrons only partially shield the increased nuclear
charge and hence the effective nuclear charge increases slightly. However, the
extra electrons added to the 3d sub shell strongly repel the 4s electrons and
these two forces are operated in opposite direction and as they tend to balance
each other, it leads to constancy in atomic radii.
At the end of the series,
d-orbitals of Zinc contain 10 electrons in which the repulsive interaction
between the charge and hence, the and atomic radius slightly increases.
Generally as we move down
a group atomic radius increases, the same trend is expected in d block elements
also. added to the 4d sub shell, the atomic radii of the than the corresponding
unexpected observation in the atomic radius of 5d elements which have nearly
same atomic radius as that of corresponding 4d elements. This is due to
lanthanoide contraction which is to be discussed later in this unit under inner
transition elements.
3. Ionization enthalpy:
Ionization energy of
transition element is intermediate between those of s and p block elements. As
we move from left to right in a transition metal series, the ionization
enthalpy increases as expected. This is due to increase in nuclear charge
corresponding to the filling of d electrons. The following figure show the
trends in ionisation enthalpy of transition elements.
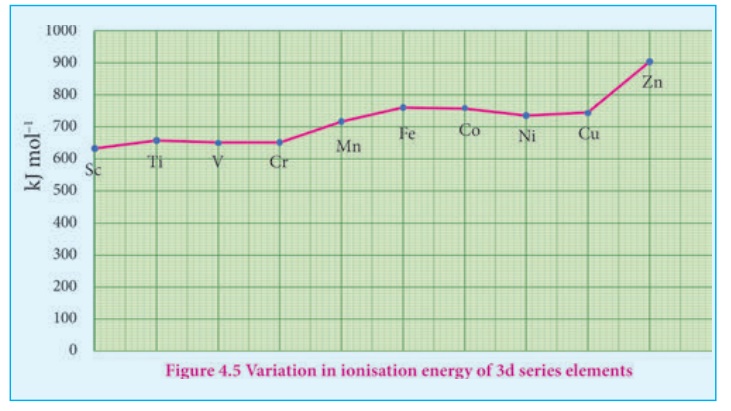
The increase in first
ionisation enthalpy with increase in atomic number along a particular series is
not regular. The added electron enters (n-1)d orbital and the inner electrons
act as a shield and decrease the effect of nuclear charge on valence ns
electrons. Therefore, it leads to variation in the ionization energy values.
The ionisation enthalpy
values can be used to predict the thermodynamic stability of their compounds.
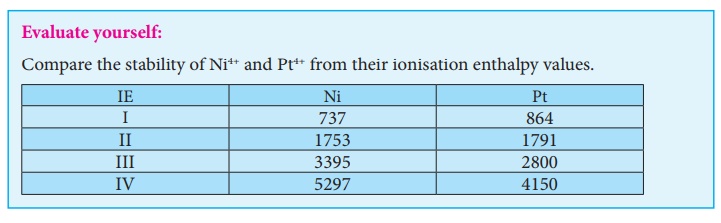
Let us compare the ionisation energy required to form Ni2+ and Pt2+ ions.
For Nickel, IE1
+ IE2 = (
737 + 1753) =
2490 kJmol−1
For Platinum, IE1
+ IE2 = (
864 + 1791) = 2655 kJmol−1
Since, the energy
required to form Ni2+ is less than that of Pt2+, Ni(II)
compounds are thermodynamically more stable than Pt(II) compounds.
4. Oxidation state:
The first transition
metal Scandium exhibits only +3 oxidation state, but all other transition
elements exhibit variable oxidation states by loosing electrons from (n-1)d
orbital and ns orbital as the energy difference between them is very small. Let
us consider the 3d series; the following table summarizes the oxidation states
of the 3d series elements.
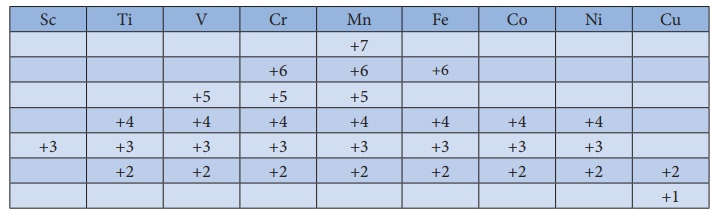
At the beginning of the
series, +3 oxidation state is stable but towards the end +2 oxidation state
becomes stable.
The number of oxidation
states increases with the number of electrons available, and it decreases as
the number of paired electrons increases. Hence, the first and last elements
show less number of oxidation states and the middle elements with more number
of oxidation states. For example, the first element Sc has only one oxidation
state +3; the middle element Mn has six different oxidation states from +2 to
+7. The last element Cu shows +1 and +2 oxidation states only.
The relative stability
of different oxidation states of 3d metals is correlated with the extra
stability of half filled and fully filled electronic configurations. Example: Mn2+
( 3d5 ) is more stable than Mn4+
( 3d3 )
The oxidation states of
4d and 5d metals vary from +3 for Y and La to +8 for Ru and Os. The highest
oxidation state of 4d and 5d elements are found in their compounds with the
higher electronegative elements like O, F and Cl. for example: RuO4,
OsO4 and WCl6. Generally in going down a group, a
stability of the higher oxidation state increases while that of lower oxidation
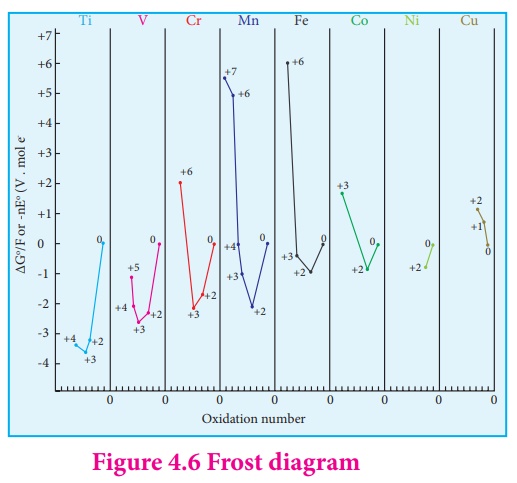
state decreases.It is evident from the Frost diagram (ΔG0 vs oxidation number)
as shown below,For titanium,vanadium and chromium, the most thermodynamically
stable oxidation state is +3. For iron, the stabilities of +3 and +2 oxidation
states are similar.Copper is unique in 3d series having a stable +1 oxidation
state. It is prone to disproportionate to the +2 and 0 oxidation states.
5. Standard electrode potentials of transition metals
Redox reactions involve
transfer of electrons from one reactant to another. Such reactions are always
coupled, which means that when one substance is oxidised, another must be
reduced. The substance which is oxidised is a reducing agent and the one which
is reduced is an oxidizing agent. The oxidizing and reducing power of an
element is measured in terms of the standard electrode potentials.
Standard electrode potential
is the value of the standard emf of a cell in which molecular hydrogen under
standard pressure ( 1atm) and temperature (273K) is oxidised to solvated
protons at the electrode.
If the standard
electrode potential (E0), of a metal is large and negative, the metal is a
powerful reducing agent, because it loses electrons easily. Standard electrode
potentials (reduction potential) of few first transition metals are given in
the following table.
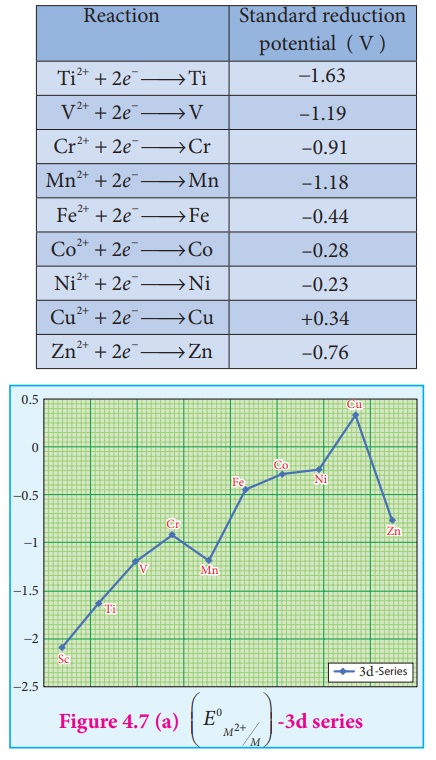
In 3d series as we move
from Ti to Zn, the standard reduction potential (E0 M2+/M)
value is approaching towards less negative value and copper has a positive
reduction potential. i.e., elemental copper is more stable than Cu2+.
There are two deviations., In the general trend, Fig shows that (E0 M2+/M)
value for manganese and zinc are more negative than the regular trend. It is
due to extra stability which arises due to the half filled d5
configuration in Mn2+ and completely filled d10
configuration in Zn2+.
Transition metals in
their high oxidation states tend to be oxidizing . For example, Fe3+ is
moderately a strong oxidant, and it oxidises copper to Cu2+ ions. The
feasibility of the reaction is predicted from the following standard electrode
potential values.
Fe3+ (aq) + e− ↔ Fe2+ E0 = 0.77V
Cu2 + (aq) + 2e− ↔ Cu(s) E0 = +0.34 V
The standard electrode
potential for the M3+ / M2+
half-cell gives the relative stability between M3+ and M2+. The reduction
potential values are tabulated as below.

The negative values for titanium, vanadium and chromium indicate that the higher oxidation state is preferred. If we want to reduce such a stable Cr3+ ion, strong reducing agent which has high negative value for reduction potential like metallic zinc ( E0 = − 0.76 V) is required.
The high reduction
potential of Mn3+/Mn2+ indicates Mn2+ is more
stable than Mn3+. For Fe3+/Fe2+ the reduction
potential is 0.77V, and this low value indicates that both Fe3+ and
Fe2+ can exist under normal
conditions. The drop from Mn to Fe is due to the electronic structure of the
ions concerned.Mn3+ has a 3d4 configuration while that of
Mn2+ is 3d5. The extra stability associated with a half
filled d sub shell makes the reduction of Mn3+ very feasible (E0 = +1.51V).
6. Magnetic properties
Most of the compounds of
transition elements are paramagnetic. Magnetic properties are related to the
electronic configuration of atoms. We have already learnt in XI STD that the
electron is spinning around its own axis, in addition to its orbital motion
around the nucleus. Due to these motions, a tiny magnetic field is generated
and it is measured in terms of magnetic moment. On the basis of magnetic
properties, materials can be broadly classified as (i) paramagnetic materials
(ii) diamagnetic materials, besides these there are ferromagnetic and
antiferromagnetic materials.
Materials with no
elementary magnetic dipoles are diamagnetic, in other words a species with all
paired electrons exhibits diamagnetism. This kind of materials are repelled by
the magnetic field because the presence of external magnetic field, a magnetic
induction is introduced to the material which generates weak magnetic field
that oppose the applied field.
Paramagnetic solids
having unpaired electrons possess magnetic dipoles which are isolated from one
another. In the absence of external magnetic field, the dipoles are arranged at
random and hence the solid shows no net magnetism. But in the presence of
magnetic field, the dipoles are aligned parallel to the direction of the
applied field and therefore, they are attracted by an external magnetic field.
Ferromagnetic materials
have domain structure and in each domain the magnetic dipoles are arranged. But
the spin dipoles of the adjacent domains are randomly oriented. Some transition
elements or ions with unpaired d electrons show ferromagnetism.
3d transition metal ions
in paramagnetic solids often have a magnetic dipole moments corresponding to
the electron spin contribution only. The orbital moment L is said to be
quenched. So the magnetic moment of the ion is given by
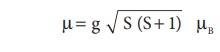
Where S is the total
spin quantum number of the unpaired electrons and is µB Bohr Magneton.
For an ion with n
unpaired electrons S =
n/2 and for an electron
g=2
Therefore the spin only
magnetic moment is given by
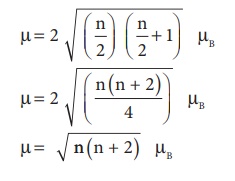
The magnetic moment
calculated using the above equation is compared with the experimental values in
the following table. In most of the cases, the agreement is good.
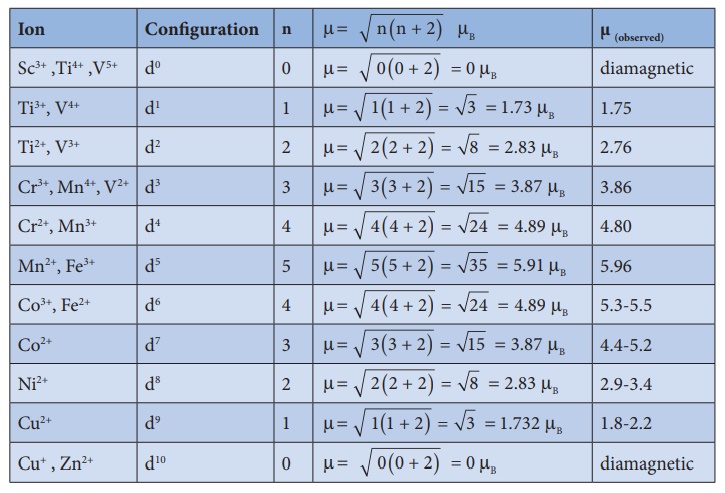
7. Catalytic properties
The chemical industries
manufacture a number of products such as polymers, flavours, drugs etc., Most
of the manufacturing processes have adverse effect on the environment so there
is an interest for eco friendly alternatives. In this context, catalyst based
manufacturing processes are advantageous, as they require low energy, minimize
waste production and enhance the conversion of reactants to products.
Many industrial
processes use transition metals or their compounds as catalysts. Transition
metal has energetically available d orbitals that can accept electrons from
reactant molecule or metal can form bond with reactant molecule using its d
electrons. For example, in the catalytic hydrogenation of an alkene, the alkene
bonds to an active site by using its π electrons with an empty d orbital of the
catalyst.
The σ bond in the hydrogen
molecule breaks, and each hydrogen atom forms a bond with a d electron on an
atom in the catalyst. The two hydrogen atoms then bond with the partially
broken π -bond in the alkene to form an alkane.
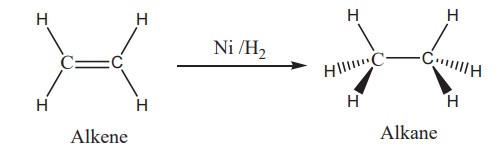
In certain catalytic
processes the variable oxidation states of transition metals find applications.
For example, in the manufacture of sulphuric acid from SO3, vanadium
pentoxide (V2O5) is used as a catalyst to oxidise SO2.
In this reaction V2O5 is reduced to vanadium (IV) Oxide
(VO2).
Some more examples are
discussed below,
(i) Hydroformylation of
olefins
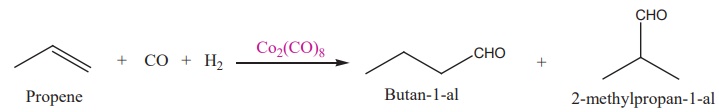
(ii) Preparation acetic
acid from acetaldehyde.

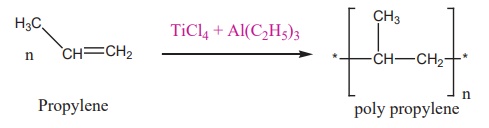
A mixture of TiCl4 and
trialkyl aluminium is used for polymerization.
(iii) Zeigler – Natta catalyst
8. Alloy formation
An alloy is formed by
blending a metal with one or more other elements. The elements may be metals or
non-metals or both. The bulk metal is named as solvent, and the other elements
in smaller portions are called solute. According to Hume-Rothery rule to form a
substitute alloy the difference between the atomic radii of solvent and solute
is less than 15%. Both the solvent and solute must have the same crystal
structure and valence and their electro negativity difference must be close to
zero. Transition metals satisfying these mentioned conditions form a number of
alloys among themselves, since their atomic sizes are similar and one metal
atom can be easily replaced by another metal atom from its crystal lattice to
form an alloy. The alloys so formed are hard and often have high melting
points. Examples: Ferrous alloys, gold – copper alloy, chrome alloys etc.,
9. Formation of interstitial compounds
An interstitial compound
or alloy is a compound that is formed when small atoms like hydrogen, boron,
carbon or nitrogen are trapped in the interstitial holes in a metal lattice.
They are usually non-stoichiometric compounds.Transition metals form a number
of interstitial compounds such as TiC, ZrH1.92 , Mn4 N
etc . The elements that occupy the metal lattice provide them new properties.
·
They are hard and show electrical land thermal conductivity
·
They have high melting points higher than those of pure metals
·
Transition metal hydrides are used as powerful reducing agents
·
Metallic carbides are chemically inert.
10. Formation of complexes
Transition elements have
a tendency to form coordination compounds with a species that has an ability to
donate an electron pair to form a coordinate covalent bond. Transition metal
ions are small and highly charged and they have vacant low energy orbitals to
accept an electron pair donated by other groups. Due to these properties,
transition metals form large number of complexes. Examples: [Fe(CN)6]4-
, [Co(NH3)6]3+ , etc..
The chemistry of
coordination compound is discussed in unit 5.
Related Topics