Consequences, Cause - lanthanoid contraction | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
lanthanoid contraction
Atomic and ionic radii:
As we move across 4f
series, the atomic and ionic radii of lanthanoids show gradual decrease with
increse in atomic number. This decrese in ionic size is called lanthanoid
contraction.
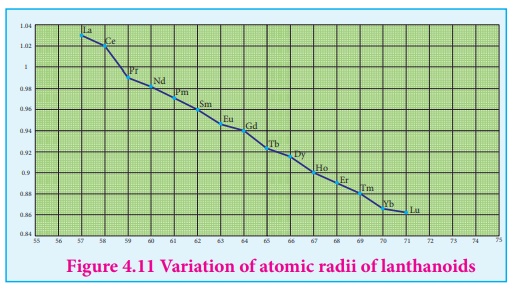
Cause of lanthanoid contraction:
As we move from one
element to another in 4f series ( Ce to Lu) the nuclear charge increases by one
unit and an additional electron is added into the same inner 4f sub shell. We
know that 4f sub shell have a diffused shapes and therefore the shielding
effect of 4f elelctrons relatively poor.hence, with increase of nuclear charge,
the valence shell is pulled slightly towards nucleus. As a result, the effetive
nuclear charge experienced by the 4f elelctorns increases and the size of Ln3+
ions decreases. Lanthanoid contraction of various lanthanoids is shown in the
graph
Consequences of lanthanoid contraction:
1. Basicity differences
As we from Ce3+
to Lu3+ , the basic character of Ln3+ ions decrease. Due
to the decrease in the size of Ln3+ ions, the ionic character of Ln −OH bond decreases
(covalent character increases) which results in the decrease in the basicity.
2. Similarities among lanthanoids:
In the complete f -
series only 10 pm decrease in atomic radii and 20 pm decrease in ionic radii is
observed. because of this very small change in radii of lanthanoids, their
chemical properties are quite similar.
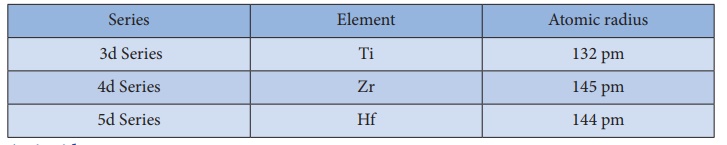
The elements of the
second and third transition series resemble each other more closely than the
elements of the first and second transition series. For example
Related Topics