Chemistry - Transition and Inner Transition Elements: Answer the following questions | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Transition and Inner Transition Elements: Answer the following questions
Chemistry : Transition and Inner Transition Elements
Answer the following questions:
1. What are transition metals? Give four examples.
• Those elements in which the last electron enters the d orbital of the penultimate shell i.e. the last electron goes to (n-l)d orbital named as d-block elements.
• These represent a transition from highly electropositive elements (metals) of s-block to least electropositive elements (non- metals) of p-block.
• Example: Cu, Au, Zn, Pt, Os
2. Explain the oxidation states of 4d series elements.
• At the beginning of the series, +3 oxidation state is stable but towards the end +2 oxidation state become stable.
• Hence the first and last elements show less number of oxidation states and the middle elements with more number of oxidation states.
4d series elements : Oxidation states
Y : +3
Ru : From +2 to +8
Cd : +2
3. What are inner transition elements?
The elements in which the extra electron enters (n-2) f orbitals are called f block elements. These elements are also called as inner transition elements because they form a transition series within the transition elements.
4. Justify the position of lanthanides and actinides in the periodic table.
Position of Lanthanides:
The actual position of Lanthanides in the periodic table is at group number 3 and period number 6.
After lanthanum, the electrons are preferentially filled in inner 4f sub shell and these fourteen elements following lanthanum show similar chemical properties.
Therefore these elements are grouped together and placed at the bottom of the periodic table.
1. General electronic configuration
[Xe] 4f1−145d0−1 6s2
2. The common oxidation state is +3
3. All these elements have similar physical and chemical properties.
Position of Actinides:
The fourteen elements following actinium resemble in their physical and chemical properties.
If these elements are place after actinium in the periodic table below 4d series, the properties of the elements belongs to a group would be different and it would affect the proper structure of the periodic table. Hence a separate position is provided to the actinides.
5. What are actinides? Give three examples.
The elements in which the extra electrons enters 5f orbitals are called actinides.
The fourteen elements following actinium are called actinoides.
Example: U, Th, Np, Pu
6. Why Gd3+ is colourless?
Gd3+: [Xe]4f7
Gd3+ exhibits exactly half filled 4f orbital. There is no electron in its outer d orbital. Hence it is colourless.
7. Explain why compounds of Cu2+ are coloured but those of Zn2+ are colourless.
Cu2+: [Ar]3d9
Zn2+: [Ar]3d10
d-d transition is responsible for color of d-block elements. Zn2+ has completely filled d orbital. Hence d-d transition is forbidden.
Cu2+ has partially filled d orbital and d-d transition is possible. Hence compounds of Cu2+ are coloured but those of Zn2+ are colourless.
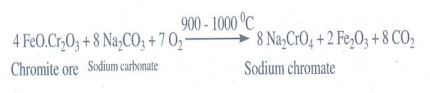
8. Describe the preparation of potassium dichromate.
i) Chromite into sodium chromate:
Potassium dichromate is prepared from chromite ore. Chromite ore is concentrated by gravity separation. It is then mixed with excess sodium carbonate and lime and roasted in a reverbratory furnace.
4 FeO.Cr2O3+ 8 Na2CO3 + 7O2 __ 900- 1000 °C___→ 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
ii) Sodium chromate into sodium dichromate:
The roasted mass is treated with water to separate soluble sodium chromate. The yellow solution of sodium chromate is treated with concentrated sulphuric acid.
2 Na2CrO4 [sodium chromate] + H2SO4 → Na2Cr2O7 [sodium dichromate] + Na2SO4 + H2O
iii) Sodium dichromate to potassium dichromate:
The above solution is concentrated and cooled.
The saturated solution of sodium dichromate in water is mixed with KCl and filtered. The filtrate is cooled to obtain K2Cr2O7.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
Na2Cr2O7 : sodium dichromate
K2Cr2O7 : potassium dichromate
9. What is lanthanide contraction and what are the effects of lanthanide contraction?
Lanthanide Contraction:
Across 4f series, Due to imperfect shielding of 4f orbital the atomic and ionic radii of lanthanides show gradual decrease with increase in atomic number. This decrease in ionic size is called lanthanide contraction.
Cause of lanthanoid contraction:
The 4f sub shell have a diffused shapes and therefore the shielding effect of 4f elelctrons relatively poor.
Hence, with increase of nuclear charge, the valence shell is pulled slightly towards nucleus.
As a result, the effective nuclear charge experienced by the 4f elelctorns increases and the size of Ln3+ ions decreases.
Consequences of lanthanide contraction:
1. Basicity differences
Ce3+ to Lu3+, the basic character of Ln3+ ions decrease. Due to the decrease in the size of Ln3+ ions, the ionic character of Ln - OH bond decreases and covalent character increases which results in the decrease in the basicity.
2. Similarities among lanthanides:
Due to small change in radii of lanthanides, their chemical properties are quite similar.
3. The elements of the second and third transition series resemble each other more closely than the elements of the first and second transition series
10. complete the following
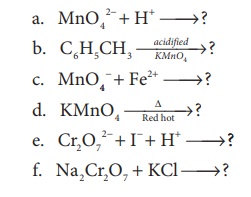
Answer:
a) 3MnO42− +4H+ → 2MnO2+ + 2H2O ↑
b) C6H5CH3 __acidified KMnO4__→ C6H5COOH
c) 2MnO −4 + 10Fe2+ + 16H+ → 2Mn2+ + 10Fe3+ + 8H2O
d) 2KMnO4 __∆_Red Hot_____→ K2MnO4 + MnO2 + O2
e) Cr2O72− + 6I− +14H+ → 2Cr3+ + 3I2 + 7H2O
f) Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
11. What are interstitial compounds?
An interstitial compound is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial holes in a metal lattice.
They are usually non-stoichiometric compounds. Transition metals form a number of interstitial compounds such as TiC, ZrH1.92, Mn4N
12. Calculate the number of unpaired electrons in Ti3+ , Mn2+ and calculate the spin only magnetic moment.
Ti: [Ar]3d24s2
Ti3+ : [Ar]3d1
Number of unpaired electron = 1
µ = √[n(n + 2)] µB
n = 1
µ = √[l(l + 2)] µB = 1.732 BM
Mn: [Ar]3d54s2
Mn2+ : [Ar]3d5
Number of unpaired electron = 5
µ = √[n(n + 2)] µB
n = 5
µ = √[5(5 + 2)] µB = 5.91 BM
13. Write the electronic configuration of Ce4+ and Co2+.
Ce4+ : [Xe]
Co2+ : [Ar]3d7
14. Explain briefly how +2 states becomes more and more stable in the first half of the first row transition elements with increasing atomic number.
In the first half, the 3d orbitals get occupied gradually as the atomic number increases. Since, the number of empty d-orbitals decreases the stability of the cations increases. The Mn2+ ion is most stable as all the five 3d - orbitals are singly occupied.
15. Which is more stable? Fe3+ or Fe2+ - explain.
Fe : [Ar]3d64s2
Fe2+ : [Ar]3d6
Fe3+ : [Ar]3d5
Fe3+ loses two electrons from 4s orbital and one electron from 3d orbital and exhibit a stable half filled 3d5 configuration, Where Fe2+ loses 2 electron from 4s orbital and shows partially filled 3d6 configuration. Hence Fe3+ is more stable than Fe2+.
16. Explain the variation in E0M3+ /M2+ 3d series.
• The standard electrode potential for the M3+/M2+ half-cell gives the relative stability between M3+ and M2+.
• The negative values for titanium, vanadium and chromium indicate that the higher oxidation state is preferred. To reduce a stable Cr3+ ion, strong reducing agent which has high negative value for reduction potential like metallic zinc ( E° = − 0.76 V) is required.
• The high reduction potential of Mn3+/ Mn2+ indicates Mn2+ is more stable than Mn3+. For Fe3+/Fe2+. the reduction potential is 0.77V, and this low value indicates that both Fe3+ and Fe2+ can exist under normal conditions. The drop from Mn to Fe is due to the electronic structure of the ions concerned. Mn3+ has a 3d4 configuration while that of Mn2+ is 3d5. The extra stability associated with a half filled d sub shell makes the reduction of Mn3+ very feasible (E° = +1.51 V)
17. Compare lanthanides and actinides.
Lanthanoids
1. Differentiating electron enters in 4f orbital
2. Binding energy of 4f orbitals are higher
3. 4f electrons have greater shielding effect.
4. They show less tendency to form complexes
5. Most of the lanthanoides are colourless
6. They do not form oxo cations
7. Besides +3 oxidation states lanthanoides show +2 and +4 oxidation states in few cases.
8. Except promethium, they are non-radioactive
Actinoides
1. Differentiating electron enters in 5f orbital
2. Binding energy of 5f orbitals are lower
3. 5f electrons have poor shielding effect
4. They show greater tendency to form complexes
5. Most of the actinoides are coloured. For example. U3+ (red), U4+ (green), UO2
6. They do form oxo cations Eg:-UO22+ , NpO22+
7. Besides +3 oxidation states actinoides show higher oxidation states such as +4, +5, +6 and +7.
8. All of them are radioactive
18. Explain why Cr2+ is strongly reducing while Mn3+ is strongly oxidizing.
Mn3+ + e− → Mn2+
Mn3+ gains one electron and converts to stable half filled Mn2+.
Hence Mn3+ is strong oxidizing agent.
Cr2+ → Cr3+ + e−
Cr2+ loses one electron and converts to Cr3+
Cr3+ ion has extra stability due to presence of half filled t2g orbital. Hence it is strong reducing agent.
19. Compare the ionization enthalpies of first series of the transition elements.
• Ionization energy of transition element is intermediate between those of s and p block elements.
• As we move from left to right the ionization enthalpy increases which is due to increase in nuclear charge corresponding to the filling of d-electrons.
• The increase in first ionisation enthalpy with increase in atomic number along a particular series is not regular. The added electron enters (n-l)d orbital and the inner electrons act as a shield and decrease the effect of nuclear charge on valence ns electrons. Therefore, it leads to variation in the ionization energy values.
• The ionisation enthalpy values can be used to predict the thermodynamic stability of their compounds.
20. Actinoid contraction is greater from element to element than the lanthanoid contraction, why?
In actinoides, 5f orbitals are filled. These 5f orbitals have a poorer shielding effect than 4f orbital.
The effective nuclear charge experienced by electrons in valence shows actinoides is much more than that experienced by lanthanoides. Hence the actinoid contraction is greater from element to element than the lanthanide.
21. Out of Lu(OH)3 and La(OH)3 which is more basic and why?
La(OH)3 is more basic than Lu(OH)3. As we move from La3+ to Lu3+, the basic character of Ln3+ ions decrease. Due to the small size of Lu3+ ions, the ionic character of Lu - OH bond decreases and covalent character increases which results in the decrease in the basicity.
22. Why europium (II) is more stable than Cerium (II)?
Eu : [Xe]4f7 5d0 6s2
Eu2+ : [Xe]4f7
Ce : [Xe]4f2 5d0 6s2
Ce2+ : [Xe]4f2
Half filled or completely filled orbitals are generally more stable.
Europium (II) shows exactly half filled orbital and stable. Cerium (II) has partially filled f orbital which is less stable than europium (II).
23. Why do zirconium and Hafnium exhibit similar properties?
Due to lanthanide contraction the second and third row transition elements shows similar atomic radii. Zr and Hf show similar atomic radii and exhibit similar chemical properties.
24. Which is stronger reducing agent Cr2+ or Fe2+?
E° value of Cr2+ / Cr is −0.91
E° value of Fe2+ /Fe is −0.44.
If the standard electrode potential (E°), of a metal is large and negative, the metal is a powerful reducing agent, because it loses electrons easily. Cr2+ is stronger reducing agent.
25. The E0M2+ /M value for copper is positive. Suggest a possible reason for this.
E° value of any metal depends on the sum of enthalpies of atomization, ionisation energies and hydration energies. Copper has high enthalpy of atomization and low hydration energy. Hence E° value of Cu2+ / Cu is positive.
26. predict which of the following will be coloured in aqueous solution Ti2+ , V3+ Sc4+, Cu+ ,Sc3+, Fe3+, Ni2+ and Co3+
Ti2+ - 3d2 -Coloured in aqueous solution
V3+ - 3d1 -Coloured in aqueous solution
Sc4+- 3d0 -No d electron. Colorless in aqueous solution
Cu+- 3d10 -Completely filled d orbital
Colourless in aqueous solution
Sc3+- 3d0 -No d electron. Colorless in aqueous solution
Fe3+- 3d5 -Coloured in aqueous solution
Ni2+ - 3d8 -Coloured in aqueous solution
Co3+- 3d6 -Coloured in aqueous solution
27. Describe the variable oxidation state of 3d series elements.
• Transition elements exhibit variable oxidation states by loosing electrons from (n−1)d orbital and ns orbital because the energy difference between them is very small.
• The number of oxidation states increases with the number of electrons available.
• Example Sc has only one oxidation state +3; the middle element Mn shows +2 to +7. The last element Cu shows +1 and +2 oxidation states only.
• The relative stability of different oxidation states of 3d metals is correlated with the extra stability of half filled and fully filled electronic configurations. Example: Mn2+ (3d5) is more stable than Mn4+ (3d3).
28. Which metal in the 3d series exhibits +1 oxidation state most frequently and why?
Cu shows +1 oxidation state more frequently in 3d series. The stable electronic configuration of Cu is [Ar]3d104s1. It loses one electron from its outer most 4s orbital and attain stable 3d10 configuration. Hence it shows +1 oxidation state.
29. Why first ionization enthalpy of chromium is lower than that of zinc?
Cr: [Ar]3d54s1
Zn : [Ar]3d104s2
• As we move from left to right in a transition metal series, the ionization enthalpy increases, due to increase in nuclear charge Nuclear charge of Zn is greater than Cr.
• The first electron removed from outermost 4s orbital. In Cr, 4s orbital was half filled where as in Zn 4s orbital was completely filled.
• Due to these reasons first ionization enthalpy of chromium is lower than that of zinc
30. Transition metals show high melting points why?
Across the period melting point first increases as the number of unpaired d electrons available for metallic bonding increases, reach a maximum value and then decreases, as the d electrons pair up and become less available for bonding. For example, in the first series the melting point increases from Scandium (m.pt 1814K) to a maximum of 2183 K for Vanadium, which is close to 2180K for Chromium. However, manganese in 3d series and Tc in 4d series have low melting point. The maximum melting point at about the middle of transition metal series indicates that d5 configuration is favorable for strong interatomic attraction.
EVALUATE YOURSELF:
1. Compare the stability of Ni4+ and Pt4+ from their ionisation enthalpy values.
IE : Ni - Pt
I : 737 - 864
II : 1753 - 1791
III : 3395 - 2800
IV : 5297 - 4150
• The ionisation enthalpy values can be used to predict the thermodynamic stability of their compounds.
• Let us compare the ionisation energy required to form Ni4+ and Pt4+ ions.
• For Nickel IEI+IEII+IEIII+IEIV =11182 kJmol−1
• For Platinum IEI+IEII+IEIII+IEIV=9605 kJmol−1
• The energy required to form Pt4+ is less than that of Ni4+. Hence Pt4+ compounds are thermodynamically more stable than Ni4+ compounds.
2. Why iron is more stable in +3 oxidation state than in +2 and the reverse is true for Manganese?
Fe2+ : [Ar]3d6
Fe3+: [Ar]3d5
Mn2+: [Ar]3d5
Mn3+: [Ar]3d4
• Fe3+ shows half filled configuration 3d5 whereas Fe2+ has 3d6 configuration. The extra stability of Fe3+ is due to its half filled electronic configuration.
• Mn2+ shows half filled configuration 3d5 whereas Mn3+ has 3d4 configuration. The extra stability of Mn2+ is due to its half filled electronic configuration.
• The stable configuration is observed in Fe3+ and Mn2+. Hence iron is more stable in +3 oxidation state than in +2 and the reverse is true for Manganese
Related Topics