Electronic configuration, Oxidation state - Actinoids | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Actinoids
Actinoids:
The fourteen elements
following actinium ,i.e., from thorium (Th) to lawrentium (Lr) are called
actinoids. Unlike the lanthanoids, all the actinoids are radioactive and most
of them have short half lives. Only thorium and uranium(U) occur in significant
amount in nature and a trace amounts of Plutonium(Pu) is also found in Uranium
ores.Neptunium(Np) and successive heavier elements are produced synthetically
by the artificial transformation of naturally occuring elements by nuclear
reactions.
Similar to lanthanoids,
they are placed at the bottom of the periodic table.
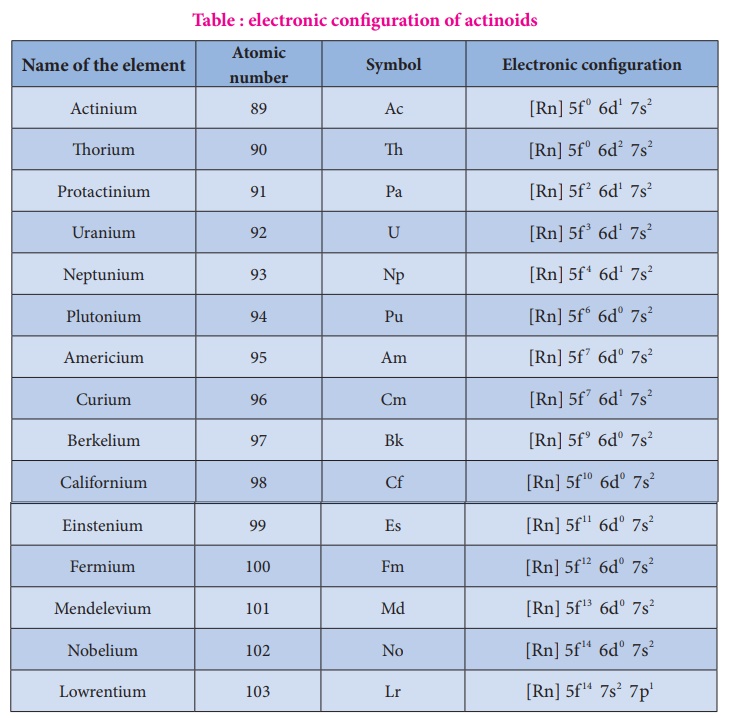
Electronic configuration:
The electronic
configuration of actinoids is not definite. The general valence shell electronic
configuration of 5f elements is represented as [Rn]5f
2 −14 6d0 −2 7s2 . The following table show the electronic
configuration of actinoids.
Table : electronic
configuration of actinoids
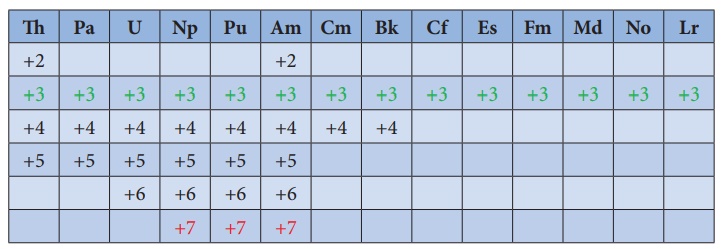
Oxidation state of actinoids:
Like lanthanoids, the
most common state of actinoids is +3. In addition to that actinoids show
variable oxidation states such as +2 , +3 , +4 ,+5,+6 and +7.
The elements
Americium(Am) and Thorium (Th) show +2 oxidation state in some compounds , for
example thorium iodide (ThI2). The elements Th , Pa, U ,Np , Pu and
Am show +5 oxidation states. Np and Pu exhibit +7 oxidation state.
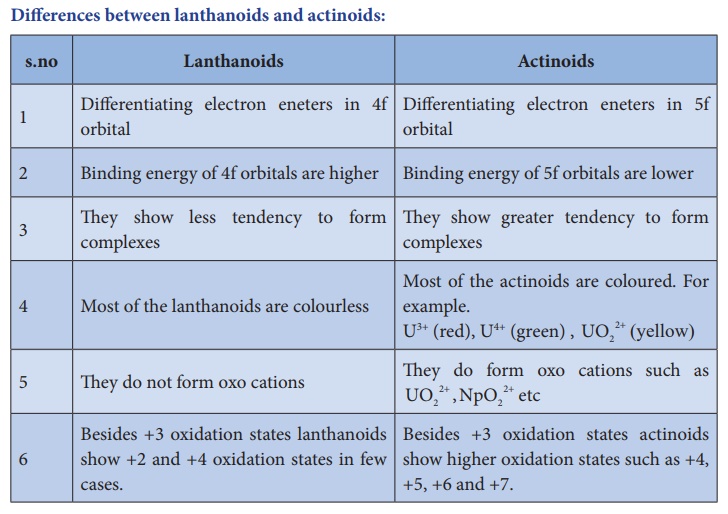
Differences between lanthanoids and actinoids:
Related Topics