Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Differences between lanthanoids and actinoids
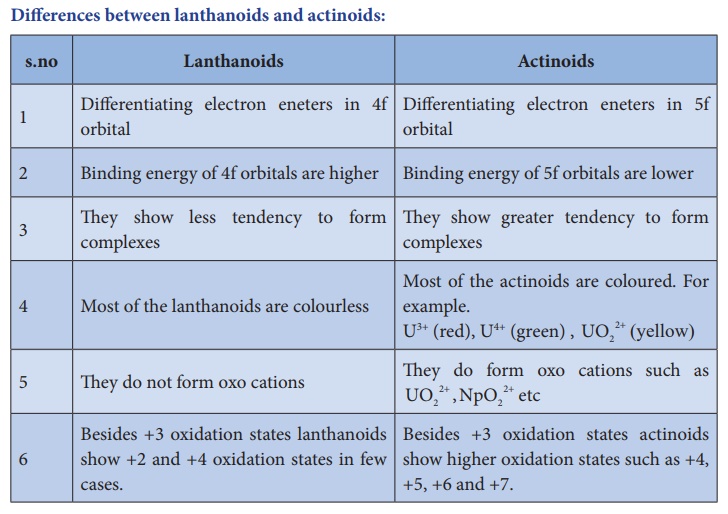
Lanthanoids
1. Differentiating electron eneters
in 4f orbital
2. Binding energy of 4f orbitals are
higher
3. They show less tendency to form
complexes
4. Most of the lanthanoids are
colourless
5. They do not form oxo cations
6. Besides +3 oxidation states
lanthanoids show +2 and +4 oxidation states in few cases.
Actinoids
1. Differentiating electron eneters
in 5f orbital
2. Binding energy of 5f orbitals are
lower
3. They show greater tendency to
form complexes
4. Most of the actinoids are coloured.
For example U3+ (red), U4+
(green) , UO22+ (yellow)
5. They do form oxo cations such as UO2+2
NpO2+2 , etc
6. Besides +3 oxidation states
actinoids show higher oxidation states such as +4, +5, +6 and +7.
Related Topics