Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
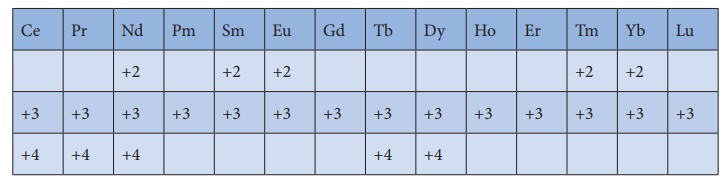
Oxidation state of lanthanoids
Oxidation state of
lanthanoids:
The common oxidation
state of lanthanoids is +3. In addition to that some of the lanthanoids also
show either +2 or +4 oxidation states.
Gd3+ and Lu3+ ions have
extra stability, it is due to the fact that they have exactly half filled and
completely filled f-orbitals respectively.their electronic c onfigurations are
Gd3+ : [ Xe ] 4 f 7
Lu3+ : [ Xe ] 4 f 14
Similarly Cerium and
terbium attain 4f0 and 4f7 configurations respectively in
the +4 oxidation states. Eu2+ and Yb2+ ions have exactly half filled
and completely filled f orbitals respectively.
The stability of
different oxidation states has an impact on the properties of these elements. the
following table shows the different oxidation states of lanthanoids.
Related Topics