Preparation, Properties, Structure, Uses - Potassium dichromate | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Potassium dichromate
Potassium
dichromate K2Cr2O7
Preparation:
Potassium dichromate is
prepared from chromate ore. The ore is concentrated by gravity separation. It
is then mixed with excess sodium carbonate and lime and roasted in a
reverbratory furnace.
4 FeCr2 O4
+ 8 Na2 CO3 + 7 O2 →900 - 10000C→ 8 Na2CrO4
+ 2 Fe2O3 + 8 CO2 ↑
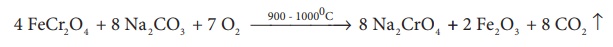
The roasted mass is
treated with water to separate soluble sodium chromate from insoluble iron
oxide. The yellow solution of sodium chromate is treated with concentrated
sulphuric acid which converts sodium chromate into sodium dichromate.
2 Na2 CrO4
+ H2SO4 →Na2Cr2O7
+ Na2SO4 + H2O
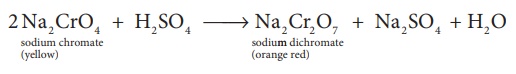
The above solution is
concentrated to remove less soluble sodium sulphate. The resulting solution is
filtered and further concentrated. It is cooled to get the crystals of Na2SO4.2H2O.
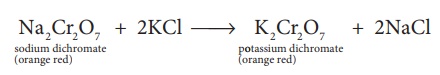
The saturated solution
of sodium dichromate in water is mixed with KCl and then concentrated to get
crystals of NaCl. It is filtered while hot and the filtrate is cooled to obtain
K2Cr2O7 crystals.
Na2 Cr2O7
+ 2KCl → K2Cr2O7
+ 2NaCl
physical properties:
Potassium dichromate is an orange red crystalline solid which melts at 671K and it is moderately soluble in cold water, but very much soluble in hot water. On heating it decomposes and forms Cr2O3 and molecular oxygen. As it emits toxic chromium fumes upon heating, it is mainly replaced by sodium dichromate.
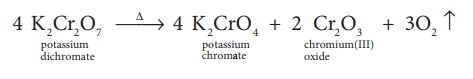
Structure of dichromate ion:
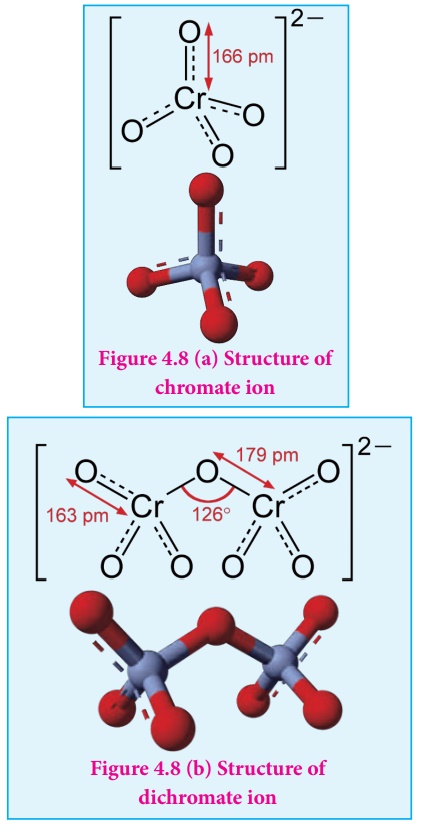
Both chromate and
dichromate ion are oxo anions of chromium and they are moderately strong
oxidizing agents. In these ions chromium is in +6 oxidation state. In an
aqueous solution, chromate and dichromate ions can be interconvertible, and in
an alkaline solution chromate ion is predominant, whereas dichromate ion
becomes predominant in acidic solutions. Structures of these ions are shown in
the figure.
Chemical properties:
1. Oxidation
Potassium dichromate is
a powerful oxidising agent in acidic medium. Its oxidising action in the
presence of H+ ions is shown below. You can note that the change in the
oxidation state of chromium from Cr6+ to Cr3+.Its
oxidising action is shown below.
Cr2O72− + 14H+ + 6e− → Cr3+ + 7 H2O
The oxidising nature of
potassium dichromate (dichromate ion) is illustrated in the following examples.
(i) It oxidises ferrous salts to ferric salts.
Cr2 O72
− + 6Fe2+ +
14H+ → 2Cr3+ + 6Fe3+
+ 7H2O
(ii) It oxidises iodide
ions to iodine
Cr2O72− + 6I− + 14H+ → 2Cr3+ + 3I2
+ 7H2O
(iii) It oxidises
sulphide ion to sulphur
Cr2O72− + 3S2− + 14H+ → 2Cr3+ + 3S +
7H2O
(iv) It oxidises sulphur
dioxide to sulphate ion
Cr2O72− + 3SO2 + 2H+ → 2Cr3+ + 3SO42− + H2O
(v) It oxidises stannous
salts to stannic salt
Cr2O72− + 3Sn2+ + 14H+ 2Cr3+ + 3Sn4+ + 7H2O
(vi) It oxidises
alcohols to acids.
2K2Cr2O7
+ 8H2SO4 + 3CH3CH2OH → 2K2SO4
+ 2Cr2 (
SO4 )3 + 3CH3COOH + 11H2O
2. Chromyl chloride test:
When potassium
dichromate is heated with any chloride salt in the presence of Conc H2SO4,
orange red vapours of chromyl chloride (CrO2Cl2) is
evolved. This reaction is used to confirm the presence of chloride ion in
inorganic qualitative analysis.
K2Cr2O7
+ 4NaCl + 6H2SO4 → 2KHSO4 + 4NaHSO4 + 2CrO2Cl2
↑ Chromyl
chloride + 3H2O

The chromyl chloride vapours are dissolved in sodium hydroxide solution and then acidified with acetic acid and treated with lead acetate. A yellow precipitate of lead chromate is obtained.
CrO2Cl2
+ 4NaOH → Na2CrO4
+ 2NaCl + 2H2O
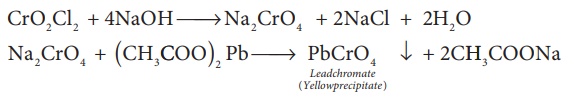
Na2CrO4
+ ( CH3COO)2 Pb → PbCrO4 ↓ (Leadchromate(Yellowprecipitate)
+ 2CH3COONa
Uses of potassium dichromate:
Some important uses of
potassium dichromate are listed below.
·
It is used as a strong oxidizing agent.
·
It is used in dyeing and printing.
·
It used in leather tanneries for chrome tanning.
·
It is used in quantitative analysis for the estimation of iron
compounds and iodides.
Related Topics