Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Electronic configuration of Lanthanoids
Electronic
configuration of Lanthanoids:
We know that the
electrons are filled in different orbitals in the order of their increasing
energy in accordance with Aufbau principle. As per this rule after filling
5s,5p and 6s and 4f level begin to fill from lanthanum, and hence the expected
electronic configuration of Lanthanum(La) is [Xe] 4f 1 5d0
6s2 but the actual electronic configuration of Lanthanum is [Xe] 4f 0
5d1 6s2 and it belongs to d block. Filling of 4f orbital
starts from Cerium (Ce) and its electronic configuration is [Xe] 4f 1
5d1 6s 2 . As we move from Cerium to other elements the
additional electrons are progressively filled in 4f orbitals as shown in the
table.
Table : electronic configuration of Lanthanum and Lanthanoids
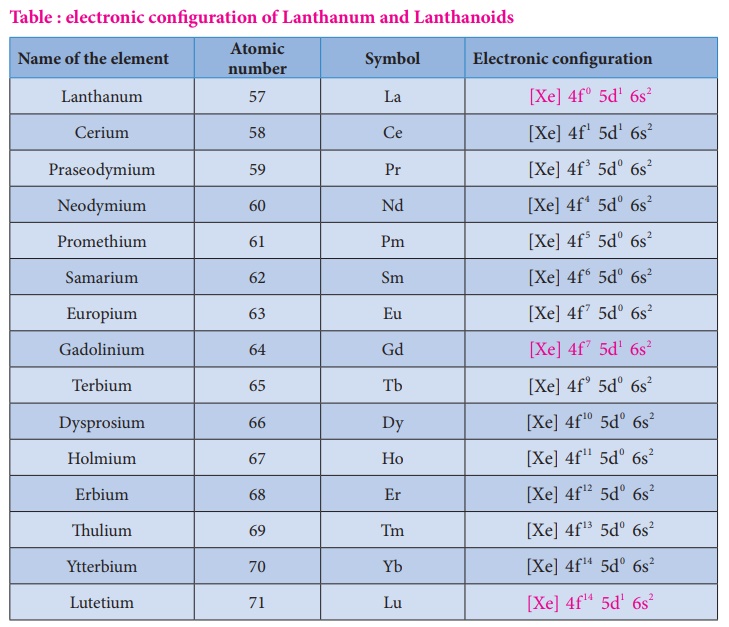
In Gadolinium (Gd) and
Lutetium (Lu) the 4f orbitals, are half-filled and completely filled, and one
electron enters 5d orbitals. Hence the general electronic configuration of 4f
series of elements can be written as [Xe] 4f 2 −14 5d0−1 6s2
Related Topics