Transition and Inner Transition Elements | Chemistry - Position of d- block elements in the periodic table | 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
Position of d- block elements in the periodic table
Position
of d- block elements in the periodic table:
We have already learnt
the periodic classification of elements in XI std. the transition metals occupy
from group –3 to group-12 of the modern periodic table.
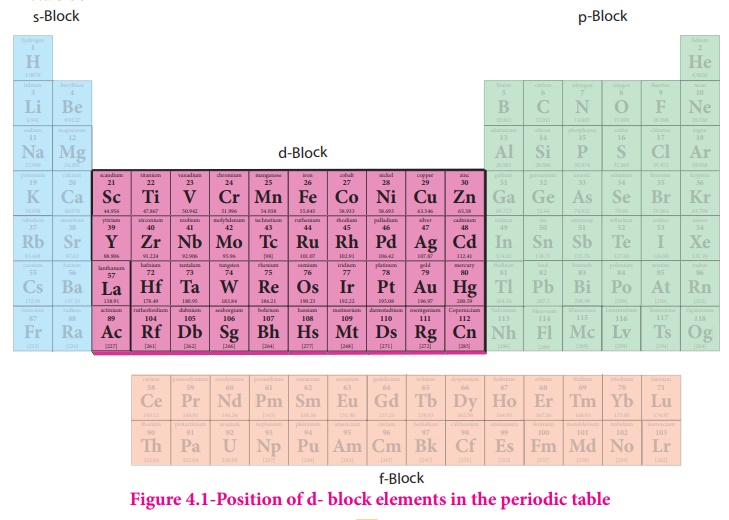
d- Block elements
composed of 3d series (4th period) Scandium to Zinc ( 10 elements), 4d series (
5th period) Yttrium to Cadmium ( 10 elements) and 5d series ( 6th period)
Lanthanum, Haffinium to mercury. As we know that the group-12 elements Zinc,
Cadmium and Mercury do not have partially filled d-orbital either in their
elemental state or in their normal oxidation states. However they are treated
as transition elements, because their properties are an extension of the
properties of the respective transition elements. As per the IUPAC definition,
the seventh period elements, starting from Ac, Rf to Cn also belong to
transition metals. All of them are radioactive. Except Actinium; all the
remaining elements are synthetically prepared and have very low half life
periods.
Related Topics