Chapter: 12th Chemistry : UNIT 4 : Transition and Inner Transition Elements
The position of Lanthanoids in the periodic table
The position of
Lanthanoids in the periodic table
The actual position of
Lanthanoids in the periodic table is at group number 3 and period
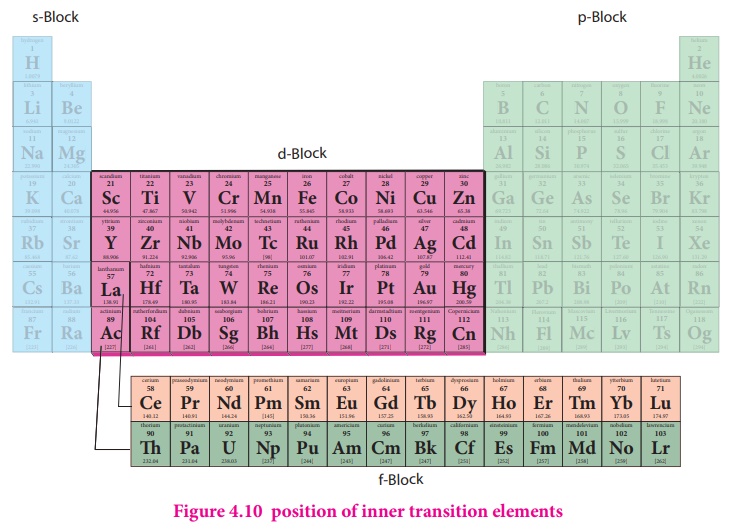
However, in the
sixth period after lanthanum, the electrons are preferentially filled in inner
4f sub shell and these fourteen elements following lanthanum show similar
chemical properties. Therefore these elements are grouped together and placed
at the bottom of the periodic table. This position can be justified as follows.
·
Lanthanoids have general electronic configuration [Xe] 4f 1−14 5d0−1 6s2
·
The common oxidation state of lanthanoides is +3
·
All these elements have similar physical and chemical properties.
Similarly the fourteen elements following actinium resemble in their physical and chemical properties. If we place these elements after Lanthanum in the periodic table below 4d series, the properties of the elements belongs to a group would be different and it would affect the proper structure of the periodic table. Hence a separate position is provided to the inner transition elements as shown in the figure.
Related Topics