Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Zeroth law of thermodynamics
Zeroth
law of thermodynamics:
The zeroth law of thermodynamics, also known as the law of
thermal equilibrium, was put forward much after the establishment of the first
and second laws of thermodynamics. It is placed before the first and second
laws as it provides a logical basis for the concept of temperature of the
system.
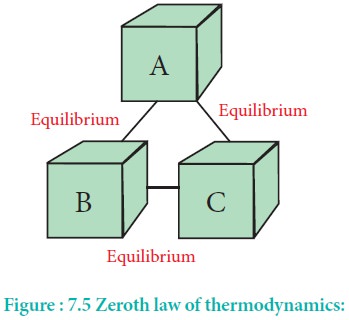
The law states that `If two systems are separately in
thermal equilibrium with a third one, then they tend to be in thermal
equilibrium with themselves'.
According to this law, if systems B and C separately are
in thermal equilibrium with another system A, then systems B and C will also be
in thermal equilibrium with each other. This is also the principle by which
thermometers are used.
Related Topics