Need, Various statements, Solved Example Problems - Second Law of thermodynamics | 11th Chemistry : UNIT 7 : Thermodynamics
Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Second Law of thermodynamics
Second Law of thermodynamics:
Need for the second law of thermodynamics:
We know from the first law of thermodynamics, the energy
of the universe is conserved. Let us consider the following processes:
1. A glass of hot water over time loses heat energy to the
surrounding and becomes cold.
2. When you mix hydrochloric acid with sodium hydroxide,
it forms sodium chloride and water with evolution of heat.
In both these processes, the total energy is conserved and
are consistent with the first law of thermodynamics. However, the reverse
process i.e. cold water becoming hot water by absorbing heat from surrounding
on its own does not occur spontaneously even though the energy change involved
in this process is also consistent with the first law. However, if the heat
energy is supplied to cold water, then it will become hot. i.e. the change that
does not occur spontaneously and an be driven by supplying energy.
Similarly, a solution of sodium chloride does not absorb
heat energy on its own, to form hydrochloric acid and sodium hydroxide. But,
this process can not be driven even by supplying energy. From these kinds of
our natural experiences, we have come to know that certain processes are spontaneous
while the others are not, and some processes have a preferred direction. In
order to explain the feasibility of a process, we need the second law of
thermodynamics.
Various statements of the second law of thermodynamics
Entropy
The second law of thermodynamics introduces another state
function called entropy. Entropy is a measure of the molecular disorder
(randomness) of a system. But thermodynamic definition of entropy is concerned
with the change in entropy that occurs as a result of a process.
It is defined as, dS = dqrev / T
Entropy statement:
The second law of thermodynamics can be expressed in terms
of entropy. i.e ŌĆ£the entropy of an isolated system increases during a
spontaneous processŌĆØ.
For an irreversible process such as spontaneous expansion
of a gas,
ŌłåStotal > 0
ŌłåStotal > ŌłåSsystem + ŌłåSsurrounding
i.e. ŌłåSuniverse > ŌłåSsystem + ŌłåSsurrounding
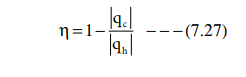
For a reversible process such as melting of ice,
ŌłåSsystem = - ŌłåSsurrounding
ŌłåSuniverse = 0
Kelvin-Planck statement:
It is impossible to construct a machine that absorbs heat
from a hot source and converts it completely into work by a cyclic process
without transferring a part of heat to a cold sink. The second law of
thermodynamics explains why even an ideal, frictionless engine cannot convert 100%
of its input heat into work. Carnot on his analysis of heat engines, found that
the maximum efficiency of a heat engine which operates reversibly, depends only
on the two temperatures between which it is operated.
Efficiency = work performed / heat absorbed
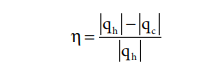
qh - heat absorbed from the hot reservoir
qc - heat transferred to cold reservoir
For a reversible cyclic process
ΔSuniverse = ΔSsystem + ΔSsurroundings
= 0
ΔSsystem = - ΔSsurroundings
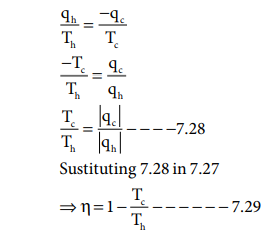
Th >> Tc
Hence, ╬Ę < 1
efficiency in percentage can be expressed as
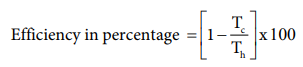
Clausius statement:
It is impossible to transfer heat from a cold reservoir to
a hot reservoir without doing some work.
Problem: 7.10
If an automobile engine burns petrol at a temperature of
816o C and if the surrounding temperature is 21o C,
calculate its maximum possible efficiency.
Solution:
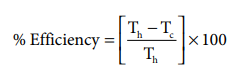
Here
Th = 816+273= 1089 K;
Tc= 21+273= 294K
%Efficiency=( 1089-294 / 1089) x100
%Efficiency=73%
Unit of entropy:
The entropy (S) is equal to heat energy exchanged (q)
divided by the temperature (T) at which the exchange takes place. Therefore,
The SI unit of entropy is JKŌłÆ1.
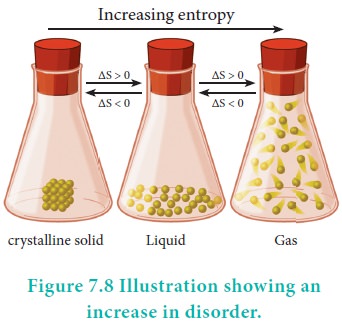
Spontaneity and Randomness
Careful examination shows that in each of the processes
viz., melting of ice and evaporation of water, there is an increase in
randomness or disorder of the system. The water molecules in ice are arranged
in a highly organised crystal pattern which permits little movement. As the ice
melts, the water molecules become disorganised and can move more freely. The
movement of molecules becomes freer in the liquid phase and even more free in
the vapour phase. In other words, we can say that the randomness of the water
molecules increases, as ice melts into water or water evaporates. Both are
spontaneous processes which result in a increase in randomness (entropy).
Standard Entropy Change(ΔS0):
It is possible to calculate the actual entropy of a
substance at any temperature above 0 K. The absolute entropy of a substance at
298 K and one bar pressure is called the standard entropy So. The
third law of thermodynamics states, according to Nernst, that the absolute
entropy of elements is zero only at 0 K in a perfect crystal, and standard
entropies of all substances at any temperature above 0 K always have positive
values. Once we know the entropies of different substances, we can calculate
the standard entropy change ( Sr0
) for chemical reactions.
S0r = Ōłæ S0products
ŌłÆ Ōłæ Sreac0 tan ts ------- (7.30)
Standard Entropy of Formation:
Standard entropy of formation is defined as ŌĆ£the entropy
of formation of 1 mole of a compound from the elements under standard
conditionsŌĆØ. It is denoted as ŌłåS0f . We can calculate the
value of entropy of a given compound from the values of S0 of
elements.
Problem: 7.6
Calculate the standard entropy change for the following
reaction( ŌłåS0f ), given the standard entropies of CO2(g),
C(s),O2(g) as 213.6 , 5.740 and 205 JKŌłÆ1 respectively.
C(g) + O2(g) ŌåÆCO2(g)
S0r = Ōłæ S0products
ŌłÆ Ōłæ Sreac0 tan ts
S0r = {S0CO 2
} ŌłÆ {SC0 + S0O2 }
S0r = 213.6 ŌłÆ [5.74 + 205]
S0r = 213.6 ŌłÆ[210.74]
S0r = 2.86 JKŌłÆ1
Entropy change accompanying change of phase
When there is a change of state from solid to liquid
(melting), liquid to vapour (evaporation) or solid to vapour (sublimation)
there is a change in entropy. This change may be carried out at constant
temperature reversibly as two phases are in equilibrium during the change.
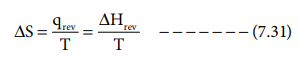
Entropy of fusion:
The heat absorbed, when one mole of a solid melts at its
melting point reversibly, is called molar heat of fusion. The entropy change
for this process is given by

where ΔHfusion is Molar heat of fusion. Tf
is the melting point.
Entropy of vapourisation:
The heat absorbed, when one mole of liquid is boiled at
its boiling point reversibly, is called molar heat of vapourisation. The
entropy change is given by
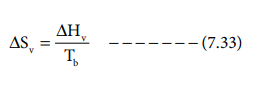
where ΔHv is Molar heat of vaporisation.
Tb is the boiling point.
Entropy of transition:
The heat change, when one mole of a solid changes
reversibly from one allotropic form to another at its transition temperature is
called enthalpy of transition. The entropy change is given
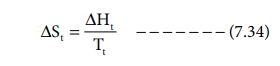
where ΔHt is the molar heat of transition, Tt
is the transition temperature.
Problem: 7.7
Calculate the entropy change during the melting of one
mole of ice into water at 00 C and 1 atm pressure. Enthalpy of fusion of ice is
6008 J mol-1
Given:
ŌłåHfusion = 6008 JmolŌłÆ1
Tf = 0 0
C = 273 K
H 2O(S) --273 KŌåÆ H 2O (
l)
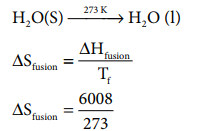
S fusion = 22 .007 J K ŌłÆ1 moleŌłÆ1
Related Topics