Solved Example Problems with Answer - Evaluate Yourself: Thermodynamics(Chemistry) | 11th Chemistry : UNIT 7 : Thermodynamics
Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Evaluate Yourself: Thermodynamics(Chemistry)
Evaluate Yourself
1. Calculate ŌłåHf┬░ for
the reaction
CO2(g)+ H2(g)
ŌåÆ CO(g)+ H2O(g)
given that ŌłåHf┬░ for CO2
(g), CO (g) and H2O(g) are ŌĆō 393.5, ŌĆō 111.31 and ŌĆō 242 kJ molŌĆō1
respectively.
Solution
:
Given
ŌłåHf0 CO2 = -393.5
kJ mol-1
ŌłåHf0 CO = -111.31 kJ mol-1
ŌłåHf0 (H2O) = -242
kJ mol-1
CO2(g) + H2(g)
ŌåÆ CO(g) +
H2O(g)
ŌłåHr0 = ?
ŌłåHr0 = ╬Ż
(ŌłåHf0 )productsŌĆō ╬Ż (ŌłåHf0)reactants
ŌłåHr0 = [ŌłåHf0
(CO) + ŌłåHf0 (H2O)] ŌĆō [ŌłåHf0
(CO2 )+ŌłåHf0
(H2)] ŌłåHr0
= [ŌĆō 111.31 + (ŌĆō 242)] ŌĆō [ŌĆō 393.5 + (0)]
ŌłåHr0 = [ŌĆō
353.31] + 393.5
ŌłåHr0 =
40.19
ŌłåHr0 = +
40.19 kJ molŌĆō1
2. Calculate the amount of heat
necessary to raise 180 g of water from 25┬░ C to 100┬░ C. Molar heat capacity of
water is 75.3 J mol-1 K-1
Solution
:
Given :
number of moles of water n
=180g / (18g mol-1 )= 10 mol
molar heat capacity of water
CP = 75.3 J KŌĆō1
molŌĆō1
T2 = 1000 C
= 373 K
T1 = 250 C=
298 K
ΔH = nCP (T2
ŌĆō T1)
╬öH = 10 mol ├Ś 75.3 J molŌĆō1 KŌĆō1 ├Ś (373 ŌĆō 298) K
ΔH = 56475 J
ΔH = 56.475 kJ
3. From the following data at
constant volume for combustion of benzene, calculate the heat of this reaction
at constant pressure condition.
C6H6(l) + 7┬Į
O2(g) ŌåÆ 6CO2 (g) + 3 H2O(l)
ΔU at 25° C = -3268.12 kJ
Solution
:
T
= 250 C = 298 K ;
ŌłåU
= ŌĆō3268.12 kJ molŌĆō1
ŌłåH
= ?
ŌłåH
= ŌłåU + ŌłångRT
ŌłåH
= ŌłåU + (np ŌĆō nr) RT
H = -3268.12+ (6 - 7/2) x 8 314 ├Ś 10 -3 x 298.
ŌłåU
= ŌĆō3268.12 + (2.5 ├Ś 8.314 ├Ś10-3 ├Ś 298)
ŌłåU
= ŌĆō 3268.12 + 6.19
ŌłåU
= ŌĆō 3261.93 kJ molŌĆō1
4. When a mole of magnesium
bromide is prepared from 1 mole of magnesium
and 1 mole of liquid bromine, 524
kJ of energy is released.
The heat of sublimation of Mg
metal is 148 kJ molŌĆō1. The heat of dissociation of bromine gas into
atoms is 193 kJ molŌĆō1. The heat of vapourisation of liquid bromine
is 31 kJ molŌĆō1. The ionisation energy of magnesium is 2187 kJ molŌĆō1
and the electron affinity of bromine is ŌĆō 662 kJ molŌĆō1. Calculate
the lattice energy of magnesium bromide.
Solution
:
Given :
Mg(S) + Br2(1) ŌåÆ
MgBr2(S) ŌłåHf0 = ŌĆō524
KJ molŌĆō1
Sublimation :
Mg(S) ŌåÆ Mg(g)
ŌłåH10 = +148 KJ molŌĆō1
Ionisation:
Mg(g) ŌåÆ Mg2+(g) + 2eŌĆō
ŌłåH20 = 2187 KJ molŌĆō1
Br2(1) ŌåÆ Br2(g)
ŌłåH30 = +31 KJ molŌĆō1
Dissociation :
Br2(g) ŌåÆ 2Br(g)
ŌłåH40 = +193 KJ molŌĆō1
Electron affinity:
Br(g) + e ŌĆō ŌåÆ Br ŌĆō (g)
ŌłåH50 = ŌĆō 331 KJ molŌĆō1
Solution :
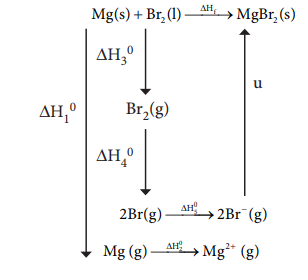
ΔHf =ΔH1 + ΔH2 + ΔH3 + ΔH4 +2ΔH5 + u
ŌĆō 524 = 148 + 2187 +
31 + 193+(2 ├Ś ŌĆō 331) + u
ŌĆō 524 = 1897 + u
u =ŌĆō524 ŌĆō 1897
u =ŌĆō 2421 kJ molŌĆō1
5. An engine operating between 1270 C and
470 C takes some specified amount of heat from a high
temperature reservoir. Assuming that there are no frictional losses, calculate
the percentage efficiency of an engine.
Solution:
Given
Th = 1270 C
= 127 + 273 = 400
K TC = 470
C = 47 + 273 = 320
K % efficiency ╬Ę = ?
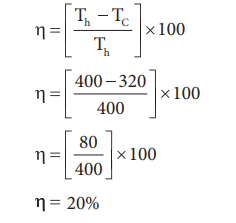
6. Urea on hydrolysis produces
ammonia and carbon dioxide. The standard entropies of urea, H2O, CO2,
NH3 are 173.8, 70, 213.5 and 192.5J mole-1K-1
respectively. Calculate the entropy change for this reaction.
Solution:
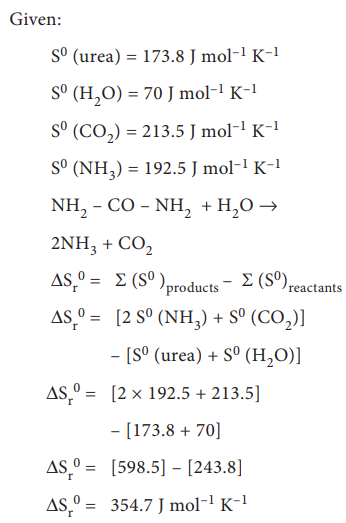
7. Calculate the entropy change
when 1 mole of ethanol is evaporated at 351 K. The molar heat of vaporisation
of ethanol is 39.84 kJ mol-1
Solution:
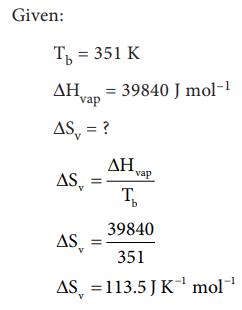
8. For a chemical reaction the
values of ╬öH and ╬öS at 300K are ŌłÆ 10 kJ moleŌłÆ1 and ŌłÆ20 J degŌłÆ1
moleŌłÆ1 respectively. What is the value of ╬öG of the reaction?
Calculate the ΔG of a reaction at 600 K assuming ΔH and ΔS values are constant.
Predict the nature of the reaction.
Solution:
Given:
ΔH
= ŌĆō10 kJ molŌĆō1 =
ŌĆō10000 J molŌĆō1
╬öS = ŌĆō 20 J KŌĆō1 molŌĆō1
T =300 K
ΔG
=?
ΔG
=╬öH ŌĆō T╬öS
╬öG = ŌĆō 10 kJ molŌĆō1 ŌĆō 300 K ├Ś (ŌĆō20x10-3)
kJ KŌĆō1 molŌĆō1
ΔG
=(ŌĆō10+6) kJ molŌĆō1
ΔG
=ŌĆō 4 kJ molŌĆō1
At
600 K
ΔG
=ŌĆō 10 kJ molŌĆō1 ŌĆō 600 K
(ŌĆō20x10-3) kJ KŌĆō1 molŌĆō1
ΔG
= (ŌĆō10 + 12) kJ molŌĆō1
ΔG
= + 2 kJ molŌĆō1
The
value of ŌłåG is negative at 300K and the reaction is spontaneous, but at 600K
the value ŌłåG becomes positive and the reaction is non spontaneous.
Related Topics