Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Properties of the Thermodynamics System
Properties of the system:
Intensive and extensive properties
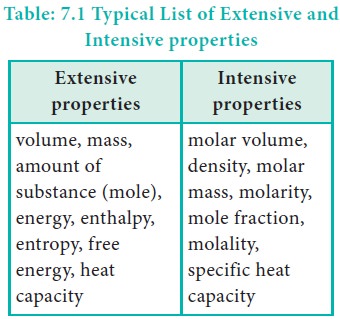
Some of the properties of a system depend on its mass or size whereas other properties do not depend on its mass or size. Based on this, the properties of a system are grouped as extensive property and intensive property.
Extensive properties:
The property that depends on the mass or the size of the system is called an extensive property.
Examples: Volume, Number of moles, Mass, Internal energy, etc.,
Intensive properties:
The property that is independent of the mass or the size of the system is called an intensive property.
Examples : Refractive index, Surface tension, density, temperature, Boiling point, Freezing point, molar volume, etc.,
Related Topics