Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
HessŌĆÖs law of constant heat Summation
HessŌĆÖs
law of constant heat Summation
We have already seen that the heat changes in chemical
reactions are equal to the difference in internal energy (ŌłåU) or heat content (ŌłåH)
of the products and reactants, depending upon whether the reaction is studied
at constant volume or constant pressure. Since ŌłåU and ŌłåH are functions of the
state of the system, the heat evolved or absorbed in a given reaction depends
only on the initial state and final state of the system and not on the path or
the steps by which the change takes place.
This generalisation is known as HessŌĆÖs law and stated as:
The enthalpy change of a reaction either at constant
volume or constant pressure is the same whether it takes place in a single or
multiple steps provided the initial and final states are same.
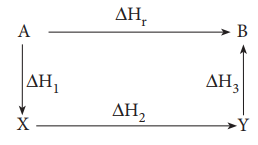
ŌłåHr = ŌłåH1 + ŌłåH2 + ŌłåH3
Application of Hess's Law:
Hess's
law can be applied to calculate enthalpies of
reactions that are difficult to measure. For example, it is very difficult to
measure the heat of combustion of graphite to give pure CO. However, enthalpy
for the oxidation of graphite to CO2 and CO to CO2 can
easily be measured. For these conversions, the heat of combustion values are
ŌĆō393.5 kJ and ŌĆō 283.5 kJ respectively.
From these data the enthalpy of combustion of graphite to
CO can be calculated by applying Hess's law.
The reactions involved in this process can be expressed as
follows
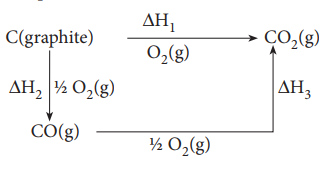
According to Hess law,
ΔH1 = ΔH2 + ΔH3
ŌĆō393.5 kJ= X ŌĆō 283.5 kJ
X= -110.5 kJ
Related Topics