with Answers and Solution - Choose the best answer: Thermodynamics | 11th Chemistry : UNIT 7 : Thermodynamics
Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Choose the best answer: Thermodynamics
Thermodynamics | Chemistry
Choose the best answer
1. The amount of heat exchanged with the surrounding at constant temperature and pressure is given by the quantity
a) ŌłåE
b) ŌłåH
c) ŌłåS
d) ŌłåG
Answer: b) ŌłåH
2. All the naturally occurring processes proceed spontaneously in a direction which leads to
a) decrease in entropy
b) increase in enthalpy
c) increase in free energy
d) decrease in free energy
Answer: d) decrease in free energy
3. In an adiabatic process, which of the following is true ?
a) q = w
b) q = 0
c) ŌłåE = q
d) P Ōłå V= 0
Answer: b) q = 0
4) In a reversible process, the change in entropy of the universe is
a) > 0
b) > 0
c) < 0
d) = 0
Answer: d) = 0
5) In an adiabatic expansion of an ideal gas
a) w = ŌĆō Ōłåu
b) w = Ōłåu + ŌłåH
c) Ōłåu = 0
d) w = 0
Answer: a) w = ŌĆō Ōłåu
6) The intensive property among the quantities below is
a) mass
b) volume
c) enthalpy
d) mass/volume
Answer: d) mass/volume
7) An ideal gas expands from the volume of 1 ├Ś 10ŌĆō3 m3 to 1 ├Ś 10ŌĆō2 m3 at 300 K against a constant pressure at 1 ├Ś 105 NmŌĆō2. The work done is
a) ŌĆō 900 J
b) 900 kJ
c) 270 kJ
d) ŌĆō 900 kJ
Answer: a) ŌĆō 900 J
Solution:
w = ŌĆō P Ōłå V
w = ŌĆō (1 ├Ś 105 NmŌĆō2)
(1├Ś 10ŌĆō2 m3 ŌĆō 1 ├Ś 10ŌĆō3 m3)
w= ŌĆō105 (10ŌĆō2 ŌĆō 10ŌĆō3) Nm
w = ŌĆō 105 (10 ŌĆō 1) 10ŌĆō3) J
w = ŌĆō 105 (9 ├Ś 10ŌĆō3) J
w = ŌĆō9 ├Ś 102 J w = ŌĆō900 J
8. Heat of combustion is always
a) positive
b) negative
c) zero
d) either positive or negative
Answer: b) negative
9. The heat of formation of CO and CO2 are ŌĆō 26.4 kCal and ŌĆō 94 kCal, respectively. Heat of combus-tion of carbon monoxide will be
a) + 26.4 kcal
b) ŌĆō 67.6 kcal
c) ŌĆō 120.6 kcal
d) + 52.8 kcal
Answer: b) ŌĆō 67.6 kcal
Solution:
CO (g )+1/2O2 (g )ŌåÆCO 2 (g)
╬öHC0 (CO) = ╬öHf (CO2) ŌĆō ╬öHf(CO) + ╬öHf (O2)]
╬öHC0 (CO) = ╬öHf (CO2) ŌĆō ╬öHf(CO) + ╬öHf (O2)]
╬öHC0 (CO) = ŌĆō 94 KCal ŌĆō [ŌĆō 26.4 KCal + 0]
╬öHC0 (CO) = ŌĆō 94 KCal + 26.4
KCal ╬öHC0 (CO) = ŌĆō 67.4 Kcal
10. C(diamond) ŌåÆ C(graphite), ŌłåH = ŌĆōve, this indicates that
a) graphite is more stable than diamond
b) graphite has more energy than diamond
c) both are equally stable
d) stability cannot be predicted
Answer: a) graphite is more stable than diamond
11. The enthalpies of formation of Al2O3 and Cr2O3 are ŌĆō 1596 kJ and ŌĆō 1134 kJ, respectively. ŌłåH for the reaction 2Al + Cr2O3 ŌåÆ 2Cr + Al2O3 is
a) ŌĆō 1365 kJ
b) 2730 kJ
c) ŌĆō 2730 kJ
d) ŌĆō 462 kJ
Answer: d) ŌĆō 462 kJ
Solution:
2Al + Cr2O3 ŌåÆ 2Cr + Al2O3
╬öHr0 = [2╬öHf (Cr) + ╬öHf (Al2O3)]ŌĆō [2╬öHf (Al) + ╬öHf
(Cr2O3)] ╬öHr0 = [0 + (ŌĆō 1596 kJ)]ŌĆō [0 + (ŌĆō 1134)]
╬öHr0 = ŌĆō 1596 kJ + 1134 kJ
╬öHr0 = ŌĆō 462 kJ
12. Which of the following is not a thermodynamic function ?
a) internal energy
b) enthalpy
c) entropy
d) frictional energy
Answer: d) frictional energy
13. If one mole of ammonia and one mole of hydrogen chloride are mixed in a closed container to form ammonium chloride gas, then
a) ŌłåH > ŌłåU
b) ŌłåH - ŌłåU = 0
c) ŌłåH + ŌłåU= 0
d) ŌłåH < ŌłåU
Answer: d) ŌłåH < ŌłåU
14. Change in internal energy, when 4 kJ of work is done on the system and 1 kJ of heat is given out by the system is
a) +1 kJ
b) ŌĆō 5 kJ
c) +3 kJ
d) ŌĆō 3 kJ
Answer: c) +3 kJ
Solution:
ΔU = q + w
╬öU = ŌĆō 1 kJ + 4 kJ
ΔU = + 3kJ
15. The work done by the liberated gas when 55.85 g of iron (molar mass 55.85 g molŌĆō1) reacts with hydrochloric acid in an open beaker at 25┬░ C
a) ŌĆō 2.48 kJ
b) ŌĆō 2.22 kJ
c) + 2.22 kJ
d) + 2.48 kJ
Answer: a) ŌĆō 2.48 kJ
Solution:
Fe + 2HCl ŌåÆ FeCl2 + H2
1 mole of Iron liberates 1 mole of
Hydrogen gas
55.85 g Iron = 1 mole Iron
Ōł┤ n = 1
T = 25┬░ C = 298 K
w = ŌĆō P ╬ö V
w =-P (nRT/P)
w = ŌĆō nRT
w = ŌĆō1 ├Ś 8.314 ├Ś 298 J
w = ŌĆō 2477.57 J
w = ŌĆō 2.48 kJ
16. The value of ŌłåH for cooling 2 moles of an ideal monatomic gas from 125┬░ C to 25┬░ C at constant pressure will be given [CP=5/2 R]
a) ŌĆō 250 R
b) ŌĆō 500 R
c) 500 R
d) + 250 R
Answer: b) ŌĆō 500 R
Solution:
Ti = 125┬░ C = 398 K
Tf = 25┬░ C = 298 K
╬öH = nCp (Tf ŌĆō Ti)
ΔH = 2x(5/2)R(298-398)
╬öH = ŌĆō 500 R
17. Given that C(g) + O2 (g) ŌåÆ CO2 (g) ŌłåH0 = ŌĆō a kJ; 2 CO(g) + O2(g) ŌåÆ 2CO2(g) ŌłåH0 = ŌĆōb kJ; Calculate the ŌłåH0 for the reaction C(g) + ┬ĮO2(g) ŌåÆ CO(g)
a) (b+2a) / 2
b) 2a - b
c) (2a-b)/2
d) (b-2a)/2
Answer: d) (b-2a)/2
Solution:
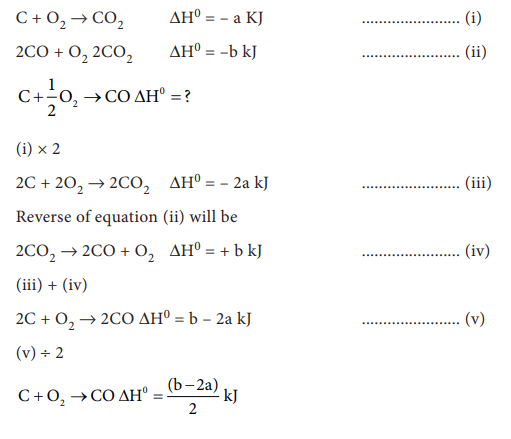
18. When 15.68 litres of a gas mixture of methane and propane are fully combusted at 00 C and 1 atmosphere, 32 litres of oxygen at the same temperature and pressure are consumed. The amount of heat of released from this combustion in kJ is (ŌłåHC (CH4) = ŌĆō 890 kJ molŌĆō1 and ŌłåHC (C3H8) = ŌĆō 2220 kJ molŌĆō1)
a) ŌĆō 889 kJ
b) ŌĆō 1390 kJ
c) ŌĆō 3180 kJ
d) ŌĆō 653.66 kJ
Answer: d) ŌĆō 653.66 kJ
Solution:
Given :
╬öHC (CH4)= ŌĆō 890 kJ molŌĆō1
╬öHC (C3H8)= ŌĆō 2220 kJ molŌĆō1

19. The bond dissociation energy of methane and ethane are 360 kJ molŌĆō1 and 620 kJ molŌĆō1 respec-tively. Then, the bond dissociation energy of C-C bond is
a) 170 kJ molŌĆō1
b) 50 kJ molŌĆō1
c) 80 kJ molŌĆō1
d) 220 kJ molŌĆō1
Answer: c) 80 kJ molŌĆō1
Solution:
4ECŌĆōH= 360 kJ molŌĆō1
ECŌĆōH= 90 kJ molŌĆō1
ECŌĆōC + 6 ECŌĆōH = 620 kJ molŌĆō1
ECŌĆōC + 6 ├Ś 90 = 620 kJ molŌĆō1
ECŌĆōC + 540= 620 kJ molŌĆō1
ECŌĆōC= 80 kJ molŌĆō1
20. The correct thermodynamic conditions for the spontaneous reaction at all temperature is
a) ŌłåH < 0 and ŌłåS > 0
b) ŌłåH < 0 and ŌłåS < 0
c) ŌłåH > 0 and ŌłåS = 0
d) ŌłåH > 0 and ŌłåS > 0
Answer: a) ŌłåH < 0 and ŌłåS > 0
21. The temperature of the system, decreases in an ___________________
a) Isothermal expansion
b) Isothermal Compression
c) adiabatic expansion
d) adiabatic compression
Answer: c) adiabatic expansion
22. In an isothermal reversible compression of an ideal gas the sign of q, ŌłåS and w are respectively
a) +, ŌĆō, ŌĆō
b) ŌĆō, +, ŌĆō
c) +, ŌĆō, +
d) ŌĆō, ŌĆō, +
Answer: d) ŌĆō, ŌĆō, +
Solution:
During compression, energy of the system increases, in isothermal condition, to main-tain temperature constant, heat is liberated from the system. Hence q is negative.
During compression entropy decreases.
During compression work is done on the system, hence w is positive
23. Molar heat of vapourisation of a liquid is 4.8 kJ molŌĆō1. If the entropy change is 16 J molŌĆō1 KŌĆō1, the boiling point of the liquid is
a) 323 K
b) 270 C
c) 164 K
d) 0.3 K
Answer: b) 270 C
Solution:
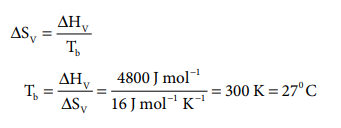
24. ŌłåS is expected to be maximum for the reaction
a) Ca(S) + ┬Į O2(g) ŌåÆ CaO(S)
b) C(S) + O2(g) ŌåÆ CO2 (g)
c) N2(g) + O2(g) ŌåÆ 2NO(g)
d) CaCO3(S) ŌåÆ CaO(S) + CO2(g)
Answer: d) CaCO3(S) ŌåÆ CaO(S) + CO2(g)
Solution:
In CaCO3(S) ŌåÆ CaO(S) + CO2(g), entropy change is positive. In (a) and (b) entropy change is negative ; in (c) entropy change is zero.
25. The values of ŌłåH and ŌłåS for a reaction are respectively 30 kJ molŌĆō1 and 100 JKŌĆō1 molŌĆō1. Then the temperature above which the reaction will become spontaneous is
a) 300 K
b) 30 K
c) 100 K
d) 200 C
Answer: a) 300 K
DG = DH ŌĆō T D S
At 300K
DG =30000 J molŌĆō1 ŌĆō 300 K x 100 J KŌĆō1 molŌĆō1
DG = 0
above 300 K ; ŌłåG will be negative and reaction becomes spontaneous.
Related Topics