Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Criteria for spontaneity of a process
Criteria for spontaneity of a process
The spontaneity of any process depends on three different factors.
· If the enthalpy change of a process is negative, then the process is exothermic and may be spontaneous. (ΔH is negative)
· If the entropy change of a process is positive, then the process may occur spontaneously. (ΔS is positive)
· The gibbs free energy which is the combination of the above two (ΔH -TΔS) should be negative for a reaction to occur spontaneously, i.e. the necessary condition for a reaction to be spontaneous is ΔH -TΔS < 0
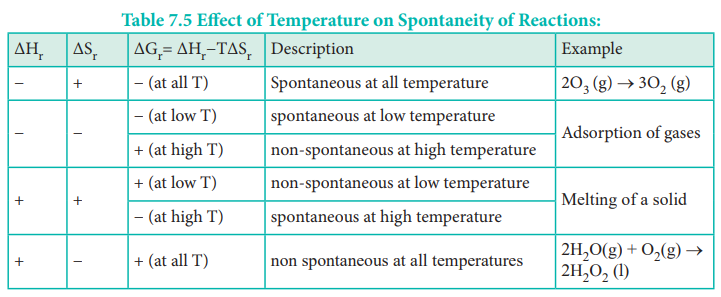
The Table assumes ΔH and ΔS will remain the way indicated for all temperatures. It may not be necessary that way. The Spontaneity of a chemical reaction is only the potential for the reaction to proceed as written. The rate of such processes is determined by kinetic factors, outside of thermodynamical prediction.
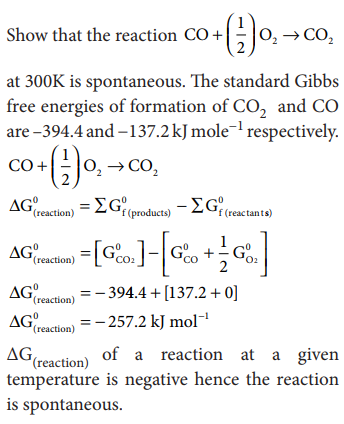
Problem: 7. 8
Related Topics