Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Gibbs free energy (G)
Gibbs
free energy (G)
One of the important applications of the second law of
thermodynamics is to predict the spontaneity of a reaction under a specific set
of conditions. A reaction that occurs under the given set of conditions without
any external driving force is called a spontaneous reaction. Otherwise, it is
said to be non-spontaneous. In our day today life, we observe many spontaneous
physical and chemical processes, which includes the following examples.
i.
A waterfall runs downhill, but never uphill,
spontaneously.
ii.
A lump of sugar dissolves spontaneously in a cup of
coffee, but never reappears in its original form spontaneously.
iii.
Heat flows from hotter object to a colder one, but never
flows from colder to hotter object spontaneously.
iv.
The expansion of a gas into an evacuated bulb is a
spontaneous process, the reverse process that is gathering of all molecules
into one bulb is not. Spontaneous
These examples show that the processes that occur
spontaneously in one direction, cannot take place in opposite direction
spontaneously.
Similarly a large number of exothermic reactions are
spontaneous. An example is combustion of methane.
CH4+2O2 ŌåÆ CO2 + 2 H2O
ΔHo =
ŌłÆ890.4 kJ molŌłÆ1
Another example is acid-base neutralization reaction:
H+ + OHŌłÆ ŌåÆ H2O
╬öHo= ŌłÆ57.32 kJ molŌłÆ1
However, some endothermic processes are also spontaneous.
For example ammonium nitrate dissolves in water spontaneously though this
dissolution is endothermic.
NH4NO3 --H2OŌåÆ NH+4
+ NOŌłÆ3
╬öHo= + 25 kJ molŌłÆ1
From the above examples we can come to the conclusion that
exothermicity favors the spontaneity but does not guarantee it. We cannot
decide whether or not a chemical reaction will occur spontaneously solely on
the basis of energy changes in the system. We know from second law of
thermodynamics that in a spontaneous process, the entropy increases. But not
all the processes which are accompanied by an increase in entropy are
spontaneous. In order to predict the spontaneity of a reaction, we need some
other thermodynamic function.
The second law of thermodynamics introduces another
thermodynamic function called Gibbs free energy which finds useful in
predicting the spontaneity of a reaction. The Gibbs free energy (G) was
developed in the 1870ŌĆÖs by Josiah Willard Gibbs. He originally termed this energy
as the ŌĆ£available energyŌĆØ to do work in a system. This quantity is the energy
associated with a chemical reaction that can be used to do work.
Gibbs free energy is defined as below
G = H - TS ------------ (7.35)
Gibbs free energy (G) is an extensive property and it is a
single valued state function.
Let us consider a system which undergoes a change of state
from state (1) to state (2) at constant temperature.
G2 ŌĆō G1 = (H2 ŌĆō H1)
ŌĆō T(S2 ŌĆō S1)
╬öG =╬öH ŌĆō T ╬öSŌłÆŌłÆŌłÆŌłÆŌłÆŌłÆ(7.36)
Now let us consider how ΔG is related to reaction
spontaneity.
We know that
ΔStotal = ΔSsys + ΔSsurr
For a reversible process (equilibrium), the change in
entropy of universe is zero. ╬öStotal = 0 [Ōł┤╬öSsys = ŌłÆ╬öSsurr]
Similarly, for an equilibrium process ΔG=0
For spontaneous process, ΔStotal > 0 , so
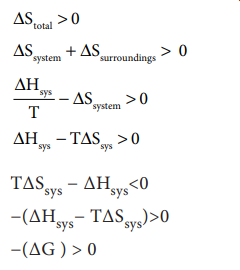
T╬öSsys ŌłÆ ╬öHsys<0
ŌłÆ(╬öHsysŌłÆ T╬öSsys)>0
ŌłÆ(╬öG ) > 0
hence for a spontaneous process,
ΔG < 0
i.e. ╬öH ŌĆō T ╬öS < 0
ŌłÆŌłÆŌłÆŌłÆŌłÆŌłÆ(7.37)
ΔHsys is the enthalpy change of a reaction, TΔSsys
is the energy which is not available to do useful work. So ΔG is the net energy
available to do useful work and is thus a measure of the ŌĆśfree energyŌĆÖ. For
this reason, it is also known as the free energy of the reaction. For non
spontaneous process, ΔG > 0
Gibbs free energy and the net work done by the system:
For any system at constant pressure and temperature
╬öG = ╬öH ŌĆō T ╬öS ŌłÆŌłÆŌłÆŌłÆ (7.36)
We know that,
ΔH =ΔU + PΔV
╬öG = ╬öU + P╬öV ŌĆō T╬öS from first law of thermodynamics
ΔU = q + w
from second law of thermodynamics
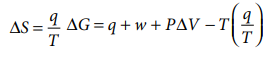
ΔG = w + PΔV
ŌłÆ╬öG = ŌłÆw ŌłÆ P╬öV ............(7.38)
But ŌłÆP╬öV represents the work done due to expansion against
a constant external pressure. Therefore, it is clear that the decrease in free
energy (ŌĆō╬öG) accompanying a process taking place at constant temperature and
pressure is equal to the maximum work obtainable from the system other than the
work of expansion.
Criteria for spontaneity of a process
The spontaneity of any process depends on three different
factors.
┬Ę
If the enthalpy change of a process is negative, then the
process is exothermic and may be spontaneous. (ΔH is negative)
┬Ę
If the entropy change of a process is positive, then the
process may occur spontaneously. (ΔS is positive)
┬Ę
The gibbs free energy which is the combination of the
above two (ΔH -TΔS) should be negative for a reaction to occur spontaneously,
i.e. the necessary condition for a reaction to be spontaneous is ΔH -TΔS < 0
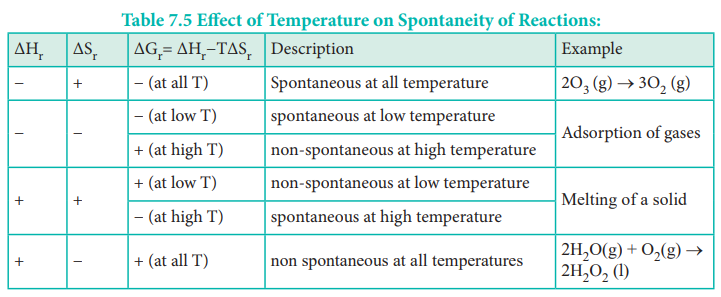
The Table assumes ΔH and ΔS will remain the way indicated
for all temperatures. It may not be necessary that way. The Spontaneity of a
chemical reaction is only the potential for the reaction to proceed as written.
The rate of such processes is determined by kinetic factors, outside of
thermodynamical prediction.
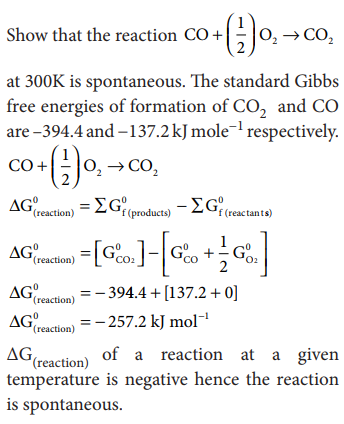
Problem: 7. 8
Relationship between standard free energy change (ΔG0) and equilibrium constant (Keq):
In a reversible process, the system is in perfect
equilibrium with its surroundings at all times. A reversible chemical reaction
can proceed in either direction simultaneously, so that a dynamic equilibrium
is set up. This means that the reactions in both the directions should proceed
with decrease in free energy, which is impossible. It is possible only if at
equilibrium, the free energy of a system is minimum. Lets consider a general equilibrium
reaction
A+B Ōćī C + D
The free energy change of the above reaction in any state
(ΔG) is related to the standard free energy change of the reaction (ΔG0
) according to the following equation.
╬öG= ╬öG0 + RT ln Q ŌłÆŌłÆŌłÆŌłÆŌłÆŌłÆ(7.39)
where Q is reaction quotient and is defined as the ratio
of concentration of the products to the concentrations of the reactants under
non equilibrium condition.
When equilibrium is attained, there is no further free
energy change i.e. ΔG 0 and Q becomes equal to equilibrium constant. Hence the
above equation becomes.
╬öG0 = ŌĆōRT ln Keq
This equation is known as VanŌĆÖt Hoff equation.
╬öG0 = ŌĆō2.303 RT log Keq
----------(7.40)
We also know that
╬öG0 = ╬öH0 ŌĆō T ╬öS0 = ŌłÆ RT
ln Keq
Problem: 7.9
Calculate ΔG0
for conversion of oxygen to ozone 3/2 O2 Ōåö O3(g) at 298 K, if Kp for this
conversion is 2.47 u 10ŌłÆ29 in standard pressure units.
Solution:
╬öG0 = ŌłÆ 2.303 RT log Kp
Where
R = 8.314 JKŌłÆ1molŌłÆ1
Kp = 2.47 x10ŌłÆ29
T = 298K
╬öG0=ŌłÆ2.303(8.314)(298)log(2.47u10ŌłÆ29)
╬öG0 = 16300 JmolŌłÆ1
╬öG0 = 16.3 KJ molŌłÆ1
Related Topics