Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Applications of the heat of combustion
Applications of the heat of combustion:
1. Calculation of heat of formation:
Since the heat of combustion of organic compounds can be determined with considerable ease, they are employed to calculate the heat of formation of other compounds.
For example let us calculate the 0 standard enthalpy of formation ΔHf of CH4 from the values of enthalpy of combustion for H2, C(graphite) and CH4 which are - 285.8, 393.5, and -890.4 kJ mol-1 respectively.
Let us interpret the information about enthalpy of formation by writing out the equations. It is important to note that the standard enthalpy of formation of pure elemental gases and elements is assumed to be zero under standard conditions. Thermochemical equation for the formation of methane from its constituent elements is,
C(graphite) + 2H2(g) ŌåÆ CH4(g)
ΔHf0 = X kJ mol-1--- (i)
Thermo chemical equations for the combustion of given substances are,
H (g ) + 1/2 ŌåÆ H2O( l)
╬öH0 = ŌĆō285.8 kJ mol-1---(ii)
C(graphite) + O2ŌåÆ CO2
╬öH0 = ŌĆō393.5 kJ mol-1--- (iii)
CH4 (g) + 2 O2ŌåÆCO2 (g)+ 2H2O (l)
╬öH0 = ŌĆō890.4 kJ mol-1--- (iv)
Since methane is in the product side of the required equation (i), we have to reverse the equation (iv)
CO2 (g)+2 H2O (l) ŌåÆ CH4 (g) + 2 O2
ΔH0 = + 890.4 kJ mol-1--- (v)
In order to get equation (i) from the remaining,
(i) = [(ii) ŌĆ” 2] + (iii) + (v)
X = [(ŌĆō285.8) ŌĆ” 2] + [ŌĆō393.5] + [+ 890.4]
= ŌĆō74.7 kJ
Hence, the amount of energy required for the formation of 1 mole of methane is -74.7 kJ
The heat of formation methane = -74.7 kJ mol-1
(2) Calculation of calorific value of food and fuels:
The calorific value is defined as the amount of heat produced in calories (or joules) when one gram of the substance is completely burnt. The SI unit of calorific value is J kgŌłÆ1. However, it is usually expressed in cal g-1.
Heat of solution:
Heat changes are usually observed when a substance is dissolved in a solvent. The heat of solution is defined as the change in enthalpy when one mole of a substance is dissolved in a specified quantity of solvent at a given temperature.
Heat of neutralisation:
The heat of neutralisation is defined as ŌĆ£The change in enthalpy when one gram equivalent of an acid is completely neutralised by one gram equivalent of a base or vice versa in dilute solutionŌĆØ.
HCl(aq)+NaOH(aq) ŌåÆ NaCl (aq)+ H2O(l)
ŌłåH = ŌĆō 57.32 kJ
H+(aq) + OH-(aq) ŌåÆ H2O(l)
ŌłåH = ŌĆō 57.32 kJ
The heat of neutralisation of a strong acid and strong base is around ŌĆō 57.32 kJ, irrespective of nature of acid or base used which is evident from the below mentioned examples.
HCl (aq) + KOH(aq) ŌåÆKCl (aq) + H2O(l)
ŌłåH = ŌĆō 57.32 kJ
HNO3(aq)+KOH(aq)ŌåÆKNO3(aq)+ H2O(l)
ŌłåH = ŌĆō 57.32 kJ
H2SO4(aq) + 2KOH(aq) ŌåÆ K2SO4(aq) + 2 H2O(l)
ŌłåH = ŌĆō 57.32 kJ
The reason for this can be explained on the basis of Arrhenius theory of acids and bases which states that strong acids and strong bases completely ionise in aqueous solution to produce H+ and OH-ions respectively. Therefore in all the above mentioned reactions the neutralisation can be expressed as follows.
H+(aq) + OH-(aq) ŌåÆ H2O(l)
ŌłåH = ŌĆō 57.32 kJ
Molar heat of fusion
The molar heat of fusion is defined as ŌĆ£the change in enthalpy when one mole of a solid substance is converted into the liquid state at its melting pointŌĆØ.
For example, the heat of fusion of ice can be represented as
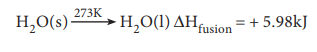
Molar heat of vapourisation
The molar heat of vaporisation is defined as ŌĆ£the change in enthalpy when one mole of liquid is converted into vapour state at its boiling pointŌĆØ.
For example, heat of vaporisation of water can be represented as
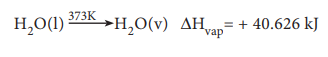
Molar heat of sublimation
Sublimation is a process when a solid changes directly into its vapour state without changing into liquid state. Molar heat of sublimation is defined as ŌĆ£the change in enthalpy when one mole of a solid is directly converted into the vapour state at its sublimation temperatureŌĆØ. For example, the heat of sublimation of iodine is represented as
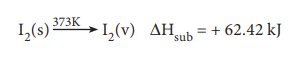
Another example of sublimation process is solid CO2 to gas at atmospheric pressure at very low temperatures.
Heat of transition
The heat of transition is defined as ŌĆ£The change in enthalpy when one mole of an element changes from one of its allotropic form to another. For example, the transition of diamond into graphite may be represented as
C(diamond) ŌåÆ C (graphite)
ŌłåHtrans= +13.81 kJ
Similarly the allotropic transitions in sulphur and phsphorous can be represented as follows,
S(monoclinic) ŌåÆS(rhombic)
ŌłåHtrans= ŌĆō 0.067 kJ
P(white) ŌåÆ P(red)
ŌłåHtrans = ŌĆō 4.301 kJ
Related Topics