Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Measurement of ΔU using bomb calorimeter
ŌłåU Measurements
For chemical reactions, heat evolved at constant volume, is measured in a bomb calorimeter.
The inner vessel (the bomb) and its cover are made of strong steel. The cover is fitted tightly to the vessel by means of metal lid and screws.
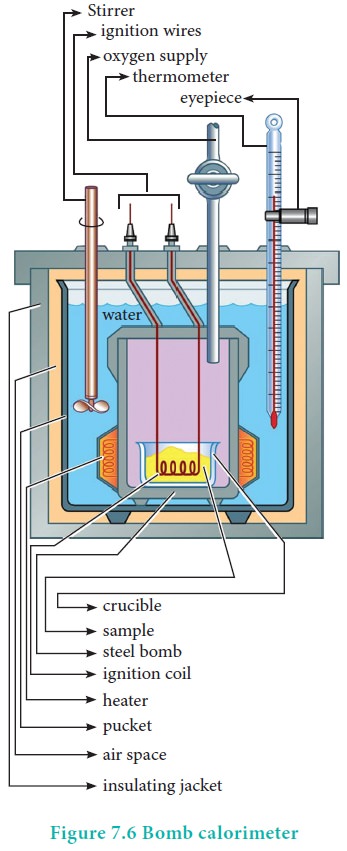
A weighed amount of the substance is taken in a platinum cup connected with electrical wires for striking an arc instantly to kindle combustion. The bomb is then tightly closed and pressurized with excess oxygen. The bomb is immersed in water, in the inner volume of the calorimeter. A stirrer is placed in the space between the wall of the calorimeter and the bomb, so that water can be stirred, uniformly. The reaction is started by striking the substance through electrical heating.
A known amount of combustible substance is burnt in oxygen in the bomb. Heat evolved during the reaction is absorbed by the calorimeter as well as the water in which the bomb is immersed. The change in temperature is measured using a Beckman thermometer. Since the bomb is sealed its volume does not change and hence the heat measurements is equal to the heat of combustion at a constant volume (ΔU)c.
The amount of heat produced in the reaction (ΔU)c is equal to the sum of the heat abosrbed by the calorimeter and water.
Heat absorbed by the calorimeter
q1 = k.ΔT
where k is a calorimeter constant equal to mc Cc ( mc is mass of the calorimeter and Cc is heat capacity of calorimeter)
Heat absorbed by the water
q2 = mw Cw ΔT
where mw is molar mass of water
Cw is molar heat capacity of water
(4,184 kJ K-1 mol-1)
Therefore ΔUc = q1 + q2
=k.ΔT + mw Cw ΔT
=(k+mw Cw)ΔT
Calorimeter constant can be determined by burning a known mass of standard sample (benzoic acid) for which the heat of combustion is known (-3227 kJmol-1)
The enthalpy of combustion at constant pressure of the substance is calculated from the equation (7.17)
ΔHC°(pressure) = ΔUC(Vol) + ΔngRT
Applications of bomb calorimeter:
1. Bomb calorimeter is used to determine the amount of heat released in combustion reaction.
2. It is used to determine the calorific value of food.
3. Bomb calorimeter is used in many industries such as metabolic study, food processing, explosive testing etc.
Related Topics