Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Solved Example Problems: Gibbs free energy (G)
Gibbs free energy (G), standard free energy change (ΔG0) and equilibrium constant (Keq)
Problem: 7. 8
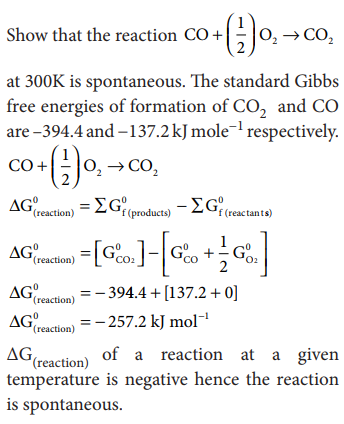
Problem: 7.9
Calculate ╬öG0 for conversion of oxygen to ozone 3/2 O2 Ōåö O3(g) at 298 K, if Kp for this conversion is 2.47 u 10ŌłÆ29 in standard pressure units.
Solution:
╬öG0 = ŌłÆ 2.303 RT log Kp
Where
R = 8.314 JKŌłÆ1molŌłÆ1
Kp = 2.47 x10ŌłÆ29
T = 298K
╬öG0=ŌłÆ2.303(8.314)(298)log(2.47u10ŌłÆ29)
╬öG0 = 16300 JmolŌłÆ1
╬öG0 = 16.3 KJ molŌłÆ1
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 7 : Thermodynamics : Solved Example Problems: Gibbs free energy (G) |
Related Topics
11th Chemistry : UNIT 7 : Thermodynamics