Chapter: 11th Chemistry : UNIT 7 : Thermodynamics
Enthalpy Changes for Different Types of Reactions and Phase Transitions
Enthalpy Changes for Different Types of Reactions and Phase Transitions:
The heat or enthalpy changes accompanying chemical reactions is expressed in different ways depending on the nature of the reaction. These are discussed below.
Standard heat of formation
The standard heat of formation of a compound is defined as ŌĆ£the change in enthalpy that takes place when one mole of a compound is formed from its elements, present in their standard states (298 K and 1 bar pressure)". By convention the standard heat of formation of all elements is assigned a value of zero.
Fe(s)+ S(s) ŌåÆ FeS(s)
╬öHf0 = ŌĆō 100.42 kJ molŌłÆ1
2C(s)+H2(g)ŌåÆ C2H2(g)
╬öHf0= + 222.33 kJ molŌłÆ1
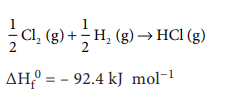
╬öHf0 = ŌĆō 92.4 kJ molŌłÆ1
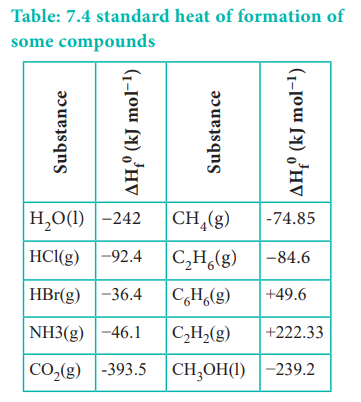
The standard heats of formation of some compounds are given in Table 7.4.
Related Topics