Chemistry - Solutions: Introduction | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Solutions: Introduction
Solutions
INTRODUCTION
There
are many chemicals that play an important role in our daily life. All these
chemicals are in different physical forms, viz solid, liquid and gas. If we do
close examination on their composition, we could find that most of them are
mixtures and rarely pure substances. One more interesting aspect is that most
of the mixtures are homogeneous irrespective of their physical state and such
homogeneous mixtures are called as solutions.
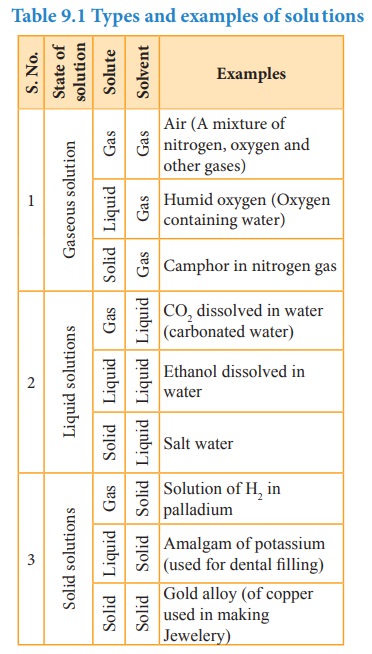
Sea
water is one of the naturally existing solutions which covers more than 70% of
the earth’s surface. We cannot imagine life on earth without sea water. It
contains many dissolved solids, mostly NaCl. Another important naturally
occurring solution is air. Air is a homogeneous mixture of nitrogen, oxygen,
carbon dioxide, and other trace gases. Even solid material such as brass is a
homogeneous mixture of copper and zinc.
In
the above examples the solutions are in different physical states viz... liquid
(sea water), gas (air) and solid (alloys), and one common property of all the
above is their homogeneity. The homogeneity implies uniform distribution of
their constituents or components throughout the mixture. In this chapter, we
learn about the solutions and their properties.
Related Topics