Chapter: 11th Chemistry : UNIT 9 : Solutions
Ideal and non-ideal solutions
Ideal and non-ideal
solutions
Ideal Solutions:
An
ideal solution is a solution in which each component i.e. the solute as well as
the solvent obeys the Raoult’s law over the entire range of concentration.
Practically no solution is ideal over the entire range of concentration.
However, when the concentration of solute is very low, the dilute solution
behaves ideally. If the two components present in the solution (A and B) are
identical in size, structure, and having almost similar intermolecular
attractive forces between them (i.e. between A-A, B-B and B-A) and then the
solution tends to behave like an ideal solution.
For an ideal solution
i)
there is no change in the volume on mixing the two components (solute &
solvents). (ΔVmixing= 0)
ii)
there is no exchange of heat when the solute is dissolved in solvent (ΔHmixing
= 0).
iii)
escaping tendency of the solute and the solvent present in it should be same as
in pure liquids.
Examples for
ideal solutions: benzene & toluene; n-hexane & n-heptane; ethyl
bromide ðyl iodide; chlorobenzene & bromobenzene.
Non-ideal solutions
The
solutions which do not obey Raoult’s law over the entire range of concentration,
are called non-ideal solutions. For a non-ideal solution, there is a change in
the volume and enthalpy upon mixing. i.e. ΔHmixing ≠0 & ΔVmixing
≠0. The deviation of the non-ideal solutions from the Raoult’s law can either
be positive or negative.
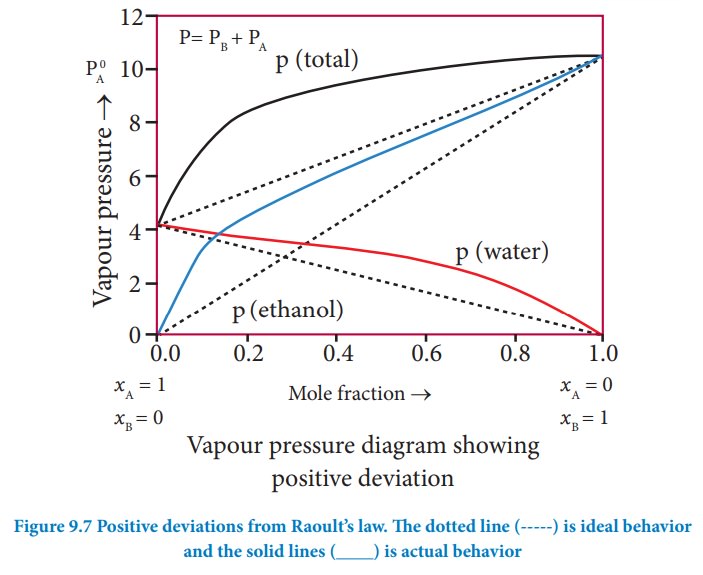
Non-ideal solutions - positive deviation from Rauolt's Law:
The
nature of the deviation from the Rauolt’s law can be explained in terms of the
intermolecular interactions between solute (A) ![]()
![]() and solvent (B). Consider a case in which the intermolecular
attractive forces between A and B are weaker than those between the molecules
of A (A-A) and molecules of B (B-B). The molecules present in such a solution
have a greater tendency to escape from the solution when compared to the ideal
solution formed by A and B, in which the intermolecular attractive forces (A-A,
B-B, A-B) are almost similar. Consequently, the vapour pressure of such
non-ideal solution increases and it is greater than the sum of the vapour
pressure of A and B as predicted by the Raoult’s law. This type of deviation is
called positive deviation.
and solvent (B). Consider a case in which the intermolecular
attractive forces between A and B are weaker than those between the molecules
of A (A-A) and molecules of B (B-B). The molecules present in such a solution
have a greater tendency to escape from the solution when compared to the ideal
solution formed by A and B, in which the intermolecular attractive forces (A-A,
B-B, A-B) are almost similar. Consequently, the vapour pressure of such
non-ideal solution increases and it is greater than the sum of the vapour
pressure of A and B as predicted by the Raoult’s law. This type of deviation is
called positive deviation.
Here,
pA > p°A xA and pB > p°B
xB
Hence
ptotal > p°A xA +
p°B xB (9.19)
Let
us understand the positive deviation by considering a solution of ethyl alcohol
and water. In this solution the hydrogen bonding interaction between ethanol
and water is weaker than those hydrogen bonding interactions amongst themselves
(ethyl alcohol-ethyl alcohol and water-water interactions). This results in the
increased evaporation of both components from the aqueous solution of ethanol.
Consequently, the vapour pressure of the solution is greater than the vapour
pressure predicted by Raoult’s law. Here, the mixing process is endothermic
i.e. ΔHmixing> 0 and there will be a slight increase in volume
(ΔVmixing> 0).
Examples for non-ideal solutions
showing positive deviations: Ethyl alcohol & cyclohexane, Benzene & acetone,
Carbon tetrachloride & chloroform, Acetone & ethyl alcohol, Ethyl alcohol & water.
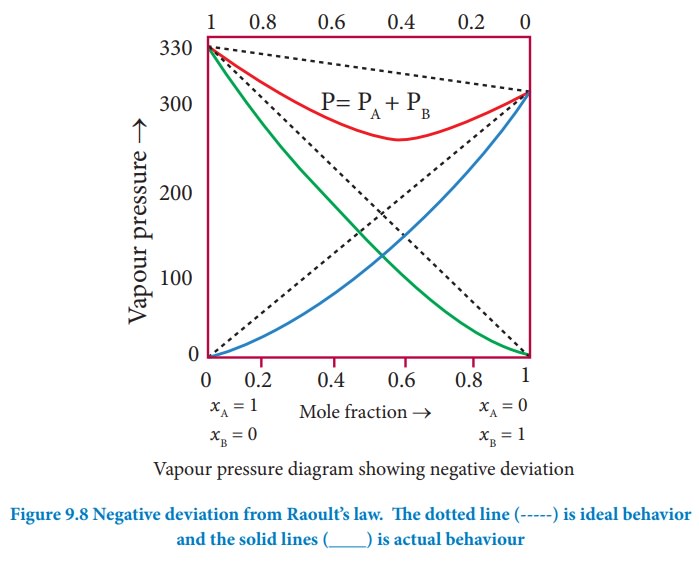
Non-ideal solutions - negative deviation from Rauolt's Law:
Let
us consider a case where the attractive forces between solute (A) and solvent
(B) are stronger than the intermolecular attractive forces between the
individual components (A-A & B-B). Here, the escaping tendency of A and B
will be lower when compared with an ideal solution formed by A and B. Hence,
the vapour pressure of such solutions will be lower than the sum of the vapour
pressure of A and B. This type of deviation is called negative deviation.
For
the negative deviation pA < p°A xA and pB
< p°B xB
Let us consider a solution of phenol and aniline. Both phenol and aniline form hydrogen bonding interactions amongst themselves. However, when mixed with aniline, the phenol molecule forms hydrogen bonding interactions with aniline, which are stronger than the hydrogen bonds formed amongst themselves. Formation of new hydrogen bonds considerably reduce the escaping tendency of phenol and aniline from the solution. As a result, the vapour pressure of the solution is less and there is a slight decrease in volume (ΔVmixing< 0) on mixing. During this process evolution of heat takes place i.e. ΔHmixing< 0 (exothermic)
Examples for non-ideal solutions
showing negative deviation:
Acetone + chloroform, Chloroform +
diethyl ether, Acetone + aniline,Chloroform + Benzene.
Factors responsible for deviation from Raoult’s law
The
deviation of solution from ideal behavior is attributed to the following
factors.
i) Solute-solvent interactions
For
an ideal solution, the interaction between the solvent molecules (A-A),the
solute molecules (B-B) and between the solvent & solute molecules (A-B) are
expected to be similar. If these interactions are dissimilar, then there will
be a deviation from ideal behavior.
ii) Dissociation of solute
When
a solute present in a solution dissociates to give its constituent ions, the
resultant ions interact strongly with the solvent and cause deviation from
Raoult’s law.
For
example, a solution of potassium chloride in water deviates from ideal behavior
because the solute dissociates to give K+ and Cl– ion
which form strong ion-dipole interaction with water molecules.
KCl
(s) + H2O (l) → K+ (aq)+ Cl– (aq)
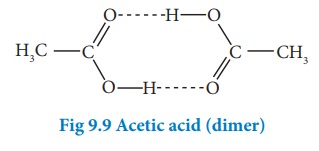
iii)Association of solute
Association
of solute molecules can also cause deviation from ideal behaviour. For example,
in solution, acetic acid exists as a dimer by forming intermolecular hydrogen
bonds, and hence deviates from Raoult’s law.
iv) Temperature
An
increase in temperature of the solution increases the average kinetic energy of
the molecules present in the solution which causes decrease in the attractive
force between them. As result, the solution deviates from ideal behaviour.
v) Pressure
At
high pressure the molecules tend to stay close to each other and therefore
there will be an increase in their intermolecular attraction. Thus, a solution
deviates from Raoult’s law at high pressure.
vi) Concentration
If
a solution is sufficiently dilute there is no pronounced solvent-solute
interaction because the number of solute molecules are very low compared to the
solvent. When the concentration is increased by adding solute, the
solvent-solute interaction becomes significant. This causes deviation from the
Raoult’s law.
Related Topics