Solutions | Chemistry - Colligative properties | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Colligative properties
Colligative
properties
Pure
water is tasteless. When you add sugar it becomes sweet, while addition of salt
makes it salty. It implies that the properties of a solution depend on the
nature of solute particles present in the solution. However, for an ideal
dilute solution, the properties, namely, relative lowering of vapour pressure,
elevation of boiling point, depression in freezing point and osmotic pressure
do not depend on the chemical nature of the solute but depends only on the
number of solute particles (ions/molecules) present in the solution. These four
properties are known as colligative properties. Though the magnitude of these
properties are small, they have plenty of practical applications. For example
the osmotic pressure is important for some vital biological systems.
Relative lowering of vapour pressure (ΔP)
The
vapour pressure of a solution containing a nonvolatile, non-electrolyte solute is always lower than the vapour pressure of the pure solvent. Consider a closed system in which a pure solvent is in equilibrium
with its vapour. At equilibrium the molar Gibbs free energies of solvent in the
liquid and gaseous phase are equal (ΔG = 0). When a solute is added to this
solvent, the dissolution takes place and its free energy (G) decreases due to
increase in entropy. In order to maintain the equilibrium, the free energy of
the vapour phase must also decrease. At a given temperature, the only way to
lower the free energy of the vapour is to reduce its pressure. Thus the vapour
pressure of the solution must decrease to maintain the equilibrium.
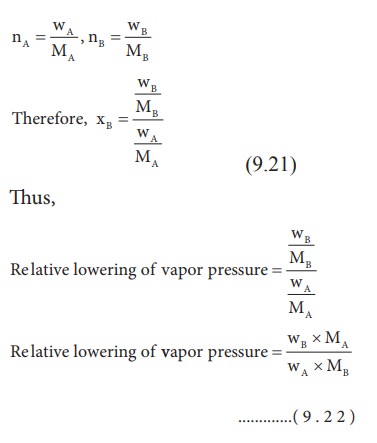
We
know that from the Raoult's law the relative lowering of the vapour pressure is
equal to the mole fraction of the solute (equation 9.10)
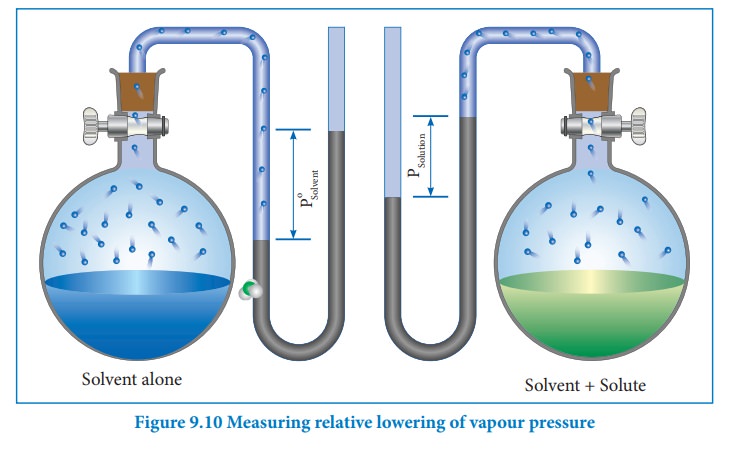
From
the above equation,it is clear that the relative lowering of vapour pressure
depends only on the mole fraction of the solute (xB) and is
independent of its nature. Therefore, relative lowering of vapour pressure is a
colligative property.
Determination of molar mass weights from relative lowering of vapour pressure
The
measurement of relative lowering of vapour pressure can be used to determine
the molar mass of a nonvolatile solute. For this purpose, a known mass of the
solute is dissolved in a known quantity of solvent. The relative lowering of
vapour pressure is measured experimentally.
According
to RaoultŌĆÖs law the relative lowering of vapor pressure is,
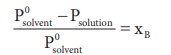
Let
w A and wB be the weights of the solvent and solute
respectively and their corresponding molar masses are MA and MB,
then the mole fraction of the solute xB is
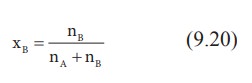
Here,
nA & nB are the moles of the solvent and the solute
respectively. For dilute solutions nA>>nB. Hence nA
+nB Ōēł nA. Now
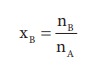
Number
of moles of solvent and the solute are,
From
the equation (9.35) the molar mass of the solute (MB) can be
calculated using the known the values of wA, wB, MA
and the measured relative lowering of vapour pressure.
Example Problem 3:
An
aqueous solution of 2 % nonvolatile solute exerts a pressure of 1.004 bar at
the boiling point of the solvent. What is the molar mass of the solute when PA┬░
is 1.013 bar?
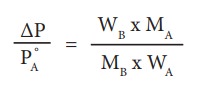
In
a 2 % solution weight of the solute is 2 g and solvent is 98 g
ΔP
= PA┬░ ŌĆō Psolution= 1.013 -1.004 bar = 0.009
bar
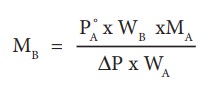
MB
= 2 x 18 x 1.013/(98 x 0.009)
=
41.3 g mol-1
Elevation of boiling point
Boiling
point is an important physical property of a liquid. The boiling point of a
liquid is the temperature at which its vapour pressure becomes equal to the
atmospheric pressure (1 atm). When a nonvolatile solute is added to a pure
solvent at its boiling point, the vapour pressure of the solution is lowered
below 1 atm. To bring the vapour pressure again to 1 atm, the temperature of
the solution has to be increased. As a result,the solution boils at a higher
temperature (Tb) than the boiling point of the pure solvent (Tb┬░).
This increase in the boiling point is known as elevation of boiling point. A
plot of vapour pressure versus temperature for water and an aqueous solution is
given below
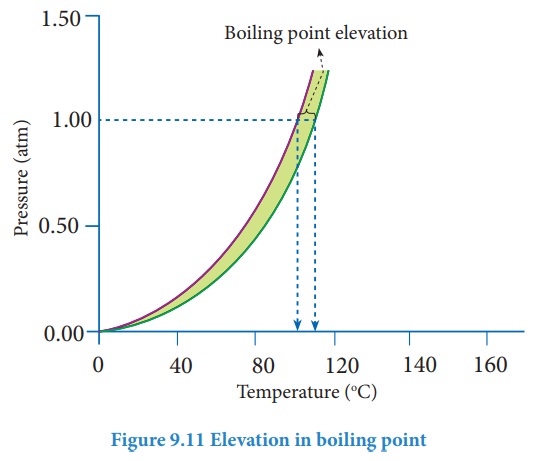
The
vapour pressure of the solution increases with increase in temperature as shown
in the above figure. The variation of vapour pressure with respect to
temperature for pure water is given by the violet coloured curve. At 100 Ōü░C the vapour pressure of water is equal
to 1 atm. Hence the boiling point of water is 100 Ōü░C (Tb┬░). When a solute is
added to water, the vapour pressure of the resultant solution is lowered. The
variation of vapour pressure with respect to temperature for the solution is
given by green curve. From the graph, it evident the vapour pressure of the
solution is equal to 1 atm pressure at the temperature Tb which is
greater than Tb┬░. The difference between these two temperatures (Tb-Tb┬░)
gives the elevation of boiling point.
The
elevation of boiling point (ΔTb)= Tb - Tb°
The
elevation of boiling point is directly proportional to the concentration of the
solute particles.
ΔTb α m (9.23)
m is the concentration of solution expressed
in molality.
ΔTb = Kb m (9.24)
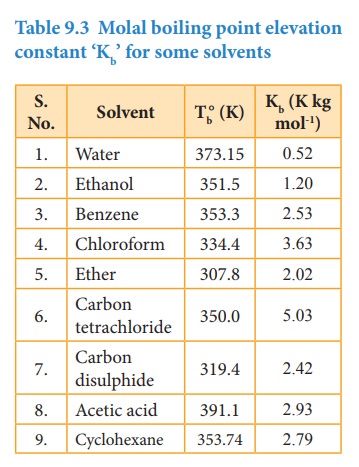
Where
Kb
= molal boiling point elevation constant or Ebullioscopic constant.
If
m=1, then ΔTb=Kb;
Hence,
the Kb is equal to the elevation in boiling point for 1 molal
solution. Kb is calculated by the following expression
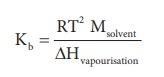
Problem:
0.75
g of an unknown substance is dissolved in 200 g water. If the elevation of
boiling point is 0.15 K and molal elevation constant is 7.5 K Kg mol-1
then, calculate the molar mass of unknown substance
ΔTb = Kb m
=
Kb x W2 x 1000 / M2 x W1
M2
= Kb x W2 x 1000 / ΔTb x W1
=
7.5 x 0.75 x 1000 / 0.15 x 200
= 187.5 g mol-1
Depression in freezing point
Freezing
point of a substance is another important physical property like boiling point.
Freezing point is defined as ŌĆ£the temperature at which the solid and the liquid
states of the substance have the same vapour pressureŌĆØ. At freezing point, the
solid and liquid phases of the substance are in equilibrium. For example, the
freezing point of water is 0 ˚C. At this temperature the ice and the water are
in equilibrium. When a nonvolatile solute is added to water at its freezing
point, the freezing point of the solution is lowered from 0 ˚C. The lowering of
the freezing point of the solvent when a solute is added is called depression
in freezing point (ΔTf).
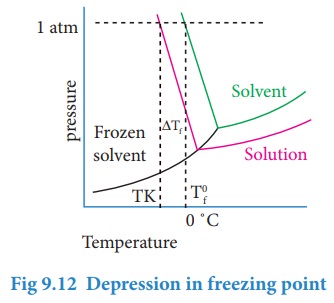
From
the above graph, we infer that the freezing point (Tfo) is 0 ˚C as the vapour pressure at this temperature is 1 atm (atmospheric
pressure). The vapour pressure versus temperature curve for the solution
indicates that the freezing point (Tf) is lower than the 0 ˚C. The
depression in freezing temperature (ΔTf) can be expressed as,
ΔTf = Tfo- Tf
The
experimental results show that the depression in freezing point is directly
proportional to the molal concentration of the solute particles.
Hence,
ΔTf
╬▒ m
ΔTf=Kf
m (9.25)
Here,
ŌĆśmŌĆÖ = is the molality of the solution
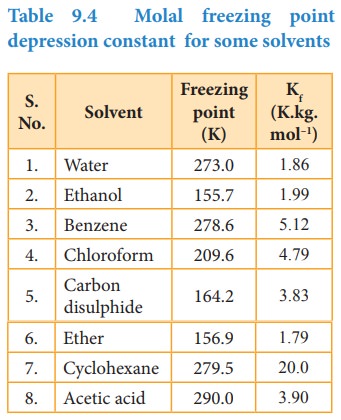
Kf
= molal freezing point depression constant or cryoscopic constant.
If
m=1 then ΔTf =Kf
The
Kf is equal to the depression in freezing point for 1 molal solution
Table 9.4 Molal
freezing point depression constant for some solvents
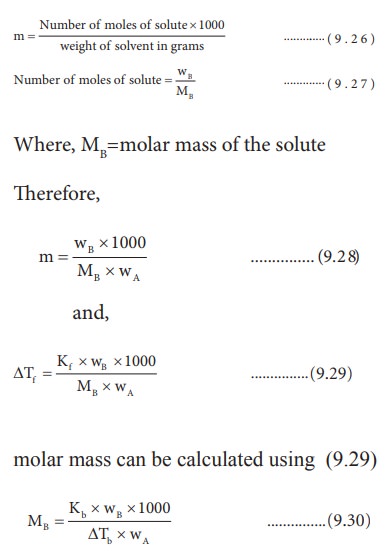
Determination of molar mass of solute from depression in freezing point
If
the solution is prepared by dissolving wB g of solute in wA
g of solvent, then the molality is,
Example Problem ŌĆō 5
Ethylene glycol (C2H6O2) can be at used as an antifreeze in the radiator of a car. Calculate the temperature when ice will begin to separate from a mixture with 20 mass percent of glycol in water used in the car radiator. Kf for water = 1.86 K Kg mol-1 and molar mass of ethylene glycol is 62 g mol-1.
Weight of solute (W2) = 20 mass percent of
solution means 20 g of ethylene glycol
Weight
of solvent (water) W1 = 100 -20 = 80 g
ΔTf
= Kfm
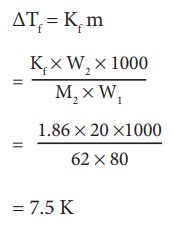
=
7.5 K
The
temperature at which the ice will begin to separate is the freezing of water
after the addition of solute i.e 7.5 K lower than the normal freezing point of
water (273-7.5K) = 265.5 K
Osmosis and osmotic pressure
Many
biological processes depend on osmosis, which is a spontaneous process by which
the solvent molecules pass through a semi permeable membrane from a solution of
lower concentration to a solution of higher concentration. The name osmosis is
derived from the Greek word ŌĆśosmosŌĆÖ
which means ŌĆśto pushŌĆÖ. It is also important to know that the semipermeable
membrane selectively allows certain molecules in the solution to pass through
it but not others.
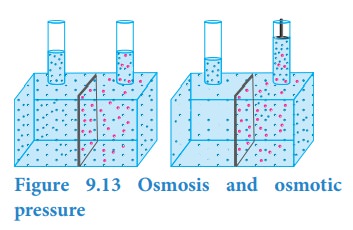
Let
us consider a simple apparatus as shown in the above figure. A semipermeable
membrane separates a chamber into two compartments. Water (pure solvent) is
added to the first compartment and the aqueous NaCl (solution) is added to the
second compartment such that the liquid levels on the both sides are equal.
Since there is a difference in concentration between the liquids present in the
two compartments, the water molecules move from first compartment to second
compartment through the semipermeable membrane. The membrane allows only the
water molecules to pass through it in either direction but not allows the NaCl.
The net flow of water is into the sodium chloride solution and hence increases
its volume. This decreases its concentration and also creates a pressure
difference between the compartments. This pressure difference, push some of the
water molecules back to the solvent side through the semipermeable membrane
until an equilibrium is established. At the equilibrium, the rate of movement
of solvent molecules on both directions are equal. The pressure difference at
the equilibrium is called osmotic pressure (ŽĆ). Thus, osmotic pressure can be
defined as ŌĆ£the pressure that must be applied to the solution to stop the
influx of the solvent (to stop osmosis) through the semipermeable membraneŌĆØ
vanŌĆÖt
Hoff found out that for dilute solutions, the osmotic pressure is directly
proportional to the molar concentration of the solute and the temperature of
the solution. He proposed the following equation to calculate osmotic pressure
which is now called as vanŌĆÖt Hoff equation.
ŽĆ=cRT
...........9.31
Here,
c
= Concentration of the solution in molarity
T
= Temperature
R
= Gas constant
Determination of molar mass from osmotic pressure
According
to vanŌĆÖt Hoff equation
ŽĆ=cRT
c=
n / V
Here,
n= number of moles of solute dissolved in ŌĆśVŌĆÖ litre of the solution.
Therefore,
ŽĆ = n/V . RT or
ŽĆV=nRT
(9.32)
If
the solution is prepared by dissolving wB g of nonvolatile solute in
wA g of solvent, then the number of moles ŌĆśnŌĆÖ is,
n=
wB/MB
Since,MB
= molar mass of the solute
Substituting
the ŌĆśnŌĆÖ in 9.32, we get,
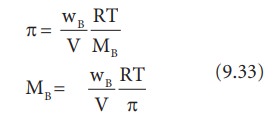
From
the equation 9.33, molar mass of the solute can be calculated.
Significances of osmotic pressure over other colligative properties
![]()
![]() Unlike elevation of boiling point (for 1 molal solution the
elevation in boiling point is 0.512 ˚C for water) and the depression in
freezing point (for 1 molal solution the depression in freezing point is 1.86
˚C for water), the magnitude of osmotic pressure is large.
Unlike elevation of boiling point (for 1 molal solution the
elevation in boiling point is 0.512 ˚C for water) and the depression in
freezing point (for 1 molal solution the depression in freezing point is 1.86
˚C for water), the magnitude of osmotic pressure is large.
The
osmotic pressure can be measured at room temperature enables to determine the
molecular mass of biomolecules which are unstable at higher temperatures.
Even
for a very dilute solution, the osmotic pressure is large.
Isotonic solutions
Two
solutions having same osmotic pressure at a given temperature are called
isotonic solutions. When such solutions are separated by a semipermeable
membrane, solvent flow between one to the other on either direction is same,
i.e. the net solvent flow between the two isotonic solutions is zero.
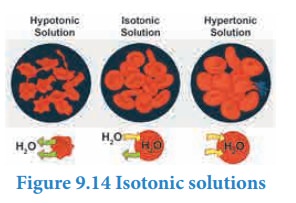
The
osmotic pressure of the blood cells is approximately equal to 7 atm at 37˚C.
The intravenous injections should have same osmotic pressure as that of the
blood (isotonic with blood). If the Intravenous solutions are too dilute that
is hypotonic, the solvent from outside of the cells will flow into the cell to
normalise the osmotic pressure and this process which is called hemolysis,
causes the cells to burst. On the other hand, if the solution is too
concentrated, that is hypertonic, the solvent molecules will flow out of the
cells, which causes the cells to shrink and die. For this reason, the
Intravenous fluids are prepared such they are isotonic to blood (0.9 % mass/
volume sodium chloride solution).
Related Topics