Chapter: 11th Chemistry : UNIT 9 : Solutions
Determination of molar mass of solute from depression in freezing point
Depression in freezing point
Freezing point of a substance is another important physical property like boiling point. Freezing point is defined as ŌĆ£the temperature at which the solid and the liquid states of the substance have the same vapour pressureŌĆØ. At freezing point, the solid and liquid phases of the substance are in equilibrium. For example, the freezing point of water is 0 ╦ÜC. At this temperature the ice and the water are in equilibrium. When a nonvolatile solute is added to water at its freezing point, the freezing point of the solution is lowered from 0 ╦ÜC. The lowering of the freezing point of the solvent when a solute is added is called depression in freezing point (╬öTf).
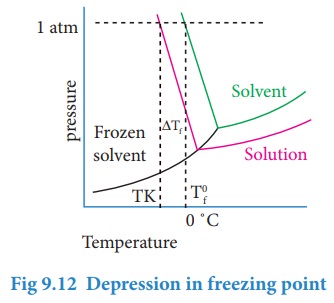
From the above graph, we infer that the freezing point (Tfo) is 0 ˚C as the vapour pressure at this temperature is 1 atm (atmospheric pressure). The vapour pressure versus temperature curve for the solution indicates that the freezing point (Tf) is lower than the 0 ˚C. The depression in freezing temperature (ΔTf) can be expressed as,
ΔTf = Tfo- Tf
The experimental results show that the depression in freezing point is directly proportional to the molal concentration of the solute particles.
Hence,
ΔTf α m
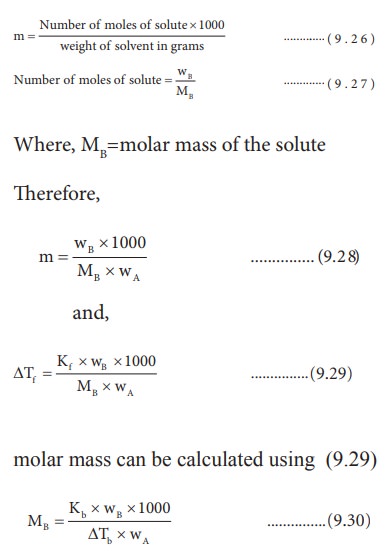
ΔTf=Kf m (9.25)
Here, ŌĆśmŌĆÖ = is the molality of the solution
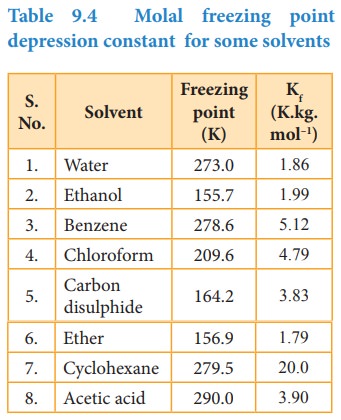
Kf = molal freezing point depression constant or cryoscopic constant.
If m=1 then ΔTf =Kf
The Kf is equal to the depression in freezing point for 1 molal solution
Table 9.4 Molal freezing point depression constant for some solvents
Determination of molar mass of solute from depression in freezing point
If the solution is prepared by dissolving wB g of solute in wA g of solvent, then the molality is,
Example Problem ŌĆō 5
Ethylene glycol (C2H6O2) can be at used as an antifreeze in the radiator of a car. Calculate the temperature when ice will begin to separate from a mixture with 20 mass percent of glycol in water used in the car radiator. Kf for water = 1.86 K Kg mol-1 and molar mass of ethylene glycol is 62 g mol-1.
Weight of solute (W2) = 20 mass percent of solution means 20 g of ethylene glycol
Weight of solvent (water) W1 = 100 -20 = 80 g
ΔTf = Kfm
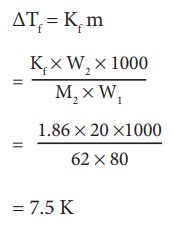
= 7.5 K
The temperature at which the ice will begin to separate is the freezing of water after the addition of solute i.e 7.5 K lower than the normal freezing point of water (273-7.5K) = 265.5 K
Related Topics