Chapter: 11th Chemistry : UNIT 9 : Solutions
Determination of molar mass from osmotic pressure
According to van’t Hoff equation
Ï€=cRT,
c= n / V
Determination of molar mass from osmotic pressure
According to van’t Hoff equation
Ï€=cRT
c= n / V
Here, n= number of moles of solute dissolved in ‘V’ litre of the solution.
Therefore, π = n/V . RT or
Ï€V=nRT (9.32)
If the solution is prepared by dissolving wB g of nonvolatile solute in wA g of solvent, then the number of moles ‘n’ is,
n= wB/MB
Since,MB = molar mass of the solute
Substituting the ‘n’ in 9.32, we get,
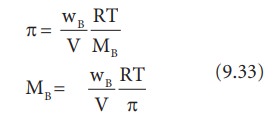
From the equation 9.33, molar mass of the solute can be calculated.
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 9 : Solutions : Determination of molar mass from osmotic pressure |
Related Topics
11th Chemistry : UNIT 9 : Solutions