Chemistry - Types of solutions | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Types of solutions
Types
of solutions
A
solution is a homogeneous mixture of two or more substances, consisting of
atoms, ions or molecules. The compound that is present in largest amount in a
homogeneous mixture is called the solvent, and the others are solutes. For
example, when a small amount of NaCl is dissolved in water, a homogeneous
solution is obtained. In this solution, Na+ and Cl- ions
are uniformly distributed in water. Here water is the solvent as the amount of
water is more compared to the amount of NaCl present in this solution, and the
NaCl is the solute.
The
commonly used solutions are the solutions in which a solid solute is dissolved
in a liquid solvent. However, solute or solvent can be in any of the three
states of matter (solid, liquid, gas). If the water is used as the solvent,the
resultant solution is called as an aqueous solution. If solvents (Benzene, CCl4,
ether etc.,) other than water is used, then the resultant solution is called as
a non-aqueous solution.
![]()
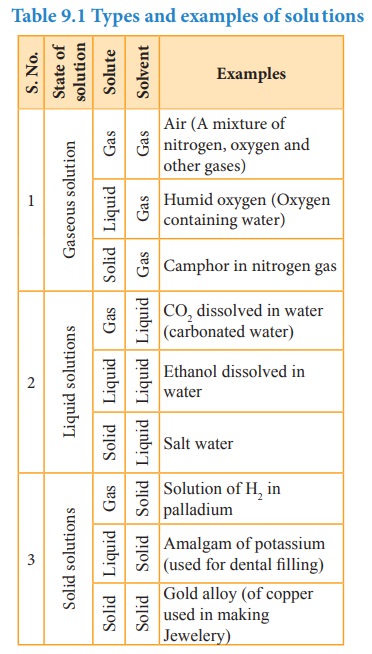
![]() The following table illustrates the different type of
solutions based on the physical state of the solute and solvent.
The following table illustrates the different type of
solutions based on the physical state of the solute and solvent.
Table 9.1 Types and
examples of solutions
Related Topics