Chemistry - Abnormal molar mass | 11th Chemistry : UNIT 9 : Solutions
Chapter: 11th Chemistry : UNIT 9 : Solutions
Abnormal molar mass
Abnormal
molar mass
The
molar masses of the nonvolatile solutes can be calculated accurately using the
experimentally determined colligative properties. In this method, we assume
that the solution is very dilute and there is no dissociation or association of
solute particles in the solution.
In
a concentrated solution, the interaction between the solute and solvent becomes
significant and reflected in the measured colligative properties. Similarly,
the dissociation or association of solute molecules would alter the total
number of particles present in the solution and hence affect the results of
measured colligative properties. In such solutions, the value of the molar mass
of the solute determined using colligative properties would be different from
the actual molar mass, and it is called abnormal molar mass.
Association or dissociation of
solute molecules:
Normally,
the molar mass determination using measured colligative properties assumes that
the solute does not dissociate or associate. In certain solvents, solute
molecules associate to form a dimer or trimer etcŌĆ” This reduces the total
number of particles (molecules) formed in solution and as a result the
calculated molar mass will be higher than the actual molar mass. Let us
consider a solution of acetic acid in benzene. It is already established that
acetic acid forms inter molecular hydrogen bonds as shown in the figure 9.9 and
exists as a dimer in benzene.
2CH3COOH
ŌåÆ(CH3COOH)2
The
molar mass of acetic acid calculated using colligative properties is found to
be around 120 g mol-1 is two times the actual molar mass (60 g mol-1).
![]()
![]() The electrolytes such as KCl or NaCl dissociates completely
into its constitutent ions in their aqueous solution. This causes an increase
in the total number of particles (ions) present in the solution. The calculated
molar mass using colligative property measurement for this type of solutions
will be lower than the actual molar mass. For example, the sodium chloride
dissociates into Na+ ions and Cl- ions in aqueous solution, as shown below.
The electrolytes such as KCl or NaCl dissociates completely
into its constitutent ions in their aqueous solution. This causes an increase
in the total number of particles (ions) present in the solution. The calculated
molar mass using colligative property measurement for this type of solutions
will be lower than the actual molar mass. For example, the sodium chloride
dissociates into Na+ ions and Cl- ions in aqueous solution, as shown below.
NaCl
(s) ŌåÆ Na+ (aq) + ClŌĆō (aq)
When
we dissolve 1 mole of NaCl (58.4 g) in water, it dissociates and gives 1 mole
of Na+ and 1 mole of Cl-. Hence, the solution will have 2
moles of particles. Thus, the colligative properties would be double the
expected value.
vanŌĆÖt Hoff factor
We
have learnt that the dissociation or association of solute molecules in a
solution will result in the increase or decrease in the calculated molar mass
using the colligative property. This variation is proportional to the extent of
association or dissociation. To quantify the extent of association or
dissociation of solutes in solution, vanŌĆÖt Hoff introduced a term 'i' which is
now called vanŌĆÖt Hoff factor. It is defined as the ratio of the actual molar
mass to the abnormal (calculated) molar mass of the solute. Here, the abnormal
molar mass is the molar mass calculated using the experimentally determined
colligative property.
i = Normal (actual) molar mass / Observed (abnormal) molar mass
= Observed colligative property / Calculated colligative property
The
estimated VanŌĆÖt Hoff factor for acetic acid solution in Benzene is 0.5 and that
of sodium chloride solution in water is 2. The degree of dissociation or
association can be related to Van't Hoff factor (i) using the following relationships
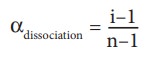
(Where
n is number ions /species formed by the dissociation of a single molecule)
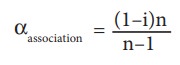
(here,
n is the number of solute involved in association.
The
equations relating the four colligative properties with the concentration of
the solutes can be rewritten as follow by incorporating the vanŌĆÖt Hoff factor
Relative
lowering of vapour pressure,
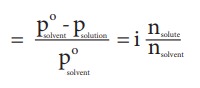
Elevation
of boiling point![]()
ŌłåTb
=iKbm
Depression
in the freezing point![]()
ŌłåTf = iKf m
Osmotic
pressure
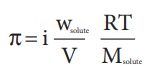
For
a solute that does not dissociate or associate the vanŌĆÖt Hoff factor is equal
to 1 (i = 1) and the molar mass will be close to the actual molar mass.
For
the solutes that associate to form higher oligomers in solution the vanŌĆÖt Hoff
factor will be less than one (i <1) and the observed molar mass will be
greater than the actual molar mass.
![]()
![]() For solutes that dissociates into their constituent ions the
vanŌĆÖt Hoff factor will be more than one (i > 1) and the observed molar mass
will be less than the normal molar mass.
For solutes that dissociates into their constituent ions the
vanŌĆÖt Hoff factor will be more than one (i > 1) and the observed molar mass
will be less than the normal molar mass.
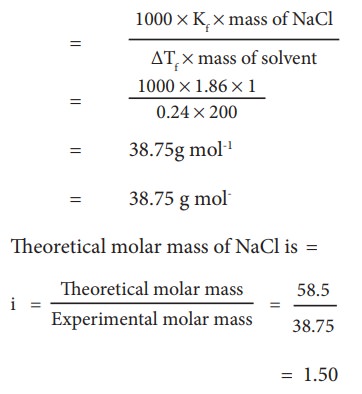
Example Problem ŌĆō 7
The
depression in freezing point is 0.24K obtained by dissolving 1g NaCl in 200g
water. Calculate vanŌĆÖt-Hoff factor. The molal depression constant is 1.86 K Kg
mol-1
Molar
mass of solute
Related Topics